Questions on Amitraz
Questions on Amitraz
Randy Oliver
ScientificBeekeeping.com
First published in ABJ March 2020
This past year, some beekeepers asked me a couple of questions about amitraz. So I ran some quick cage trials to obtain answers. The results surprised me.
Amitraz is a highly-effective acaricide (something that kills mites and ticks), and is widely used by beekeepers to control varroa. Some scofflaws apply it in the form of Taktic cattle dip, others via the approved time-release product Apivar. Amitraz has been consistently used by commercial beekeepers for two decades, and some are wondering whether mites are finally starting to develop resistance to it, as they did rather rapidly to fluvalinate and coumaphos. This question is being currently addressed by several researchers, and we should have an answer fairly soon. But this article is not about resistance, but rather two other questions posed to me by beekeepers.
Question number one: Does amitraz have any vapor action?
The first question was whether amitraz works purely by direct contact, or does it reach the mites by vapor action? If by direct contact only, then distribution of the chemical within the hive would be from bees rubbing against the strip (or other application matrix), with subsequent contact to the mite from a bee rubbing a contaminated leg over the mite, or the mite walking over molecules of amitraz on a bee’s cuticle. And since amitraz is soluble in beeswax, mites could be exposed to it (or its degradation products) there. Anyway, amitraz is generally considered to be a “contact miticide,” as stated by VetoPharma:
“Apivar works by contact: the active ingredient is delivered continuously over time. As bees walk on the strip’s surface they pick up molecules of the active ingredient and then distribute them throughout the colony.”
I assumed that vapor action was improbable, due to the extremely low vapor pressure of amitraz. Vapor pressure affects a substance’s evaporation rate, or how readily molecules of a solid or liquid will break free and diffuse as vapor into the air. Vapor pressure is typically measured by how high the vapors will push a column of mercury (mm of Hg) at any given temperature (20 C being the standard). To evaporate, any molecule must push against the atmospheric pressure of air – air pressing back at a pressure of 760 mm Hg at sea level.
As you can see in Table 1, substances with a higher vapor pressure than surrounding air evaporate rapidly; those with lower vapor pressures, more slowly. Amitraz would be expected to exhibit negligible evaporation.
| Table 1. Vapor pressures of some common substances. | |
| Substance | Vapor pressure at 20 C in mm Hg |
| Butane | >1500 |
| Air pressure at 2500 feet altitude | 694 |
| Ethanol | 44 |
| Water | 17 |
| Paraffin wax | 0.1 |
| Vaseline | 0.01 |
| Amitraz | 0.000003 |
Question number two: Does amitraz degrade rapidly?
The product information for Apivar states that:
“the strips should be used as soon as possible after opening the packaging. … Further, we recommend that opened Apivar packages not be stored for more than two weeks and that the strips be used as soon as possible.” [[1]]
Beekeepers have questioned me as to how quickly the amitraz in either Apivar strips or other application methods degrades.
Experiments to answer the questions
It occurred to me that I could answer both the vaporization action and degradation questions by running some “quick and dirty” cage trials. I had unused queen cell incubators on hand, and could use a hybrid of my mite wash cups crossed with the version of Dr. Jay Evans’ cup cages that I’d developed [[2]]. I also had a few colonies with very high mite infestation rates on hand, which would allow for having enough mites in a small sample of bees to obtain meaningful data [[3]]. And by luck I had on hand a few well-used Apivar strips that had inadvertently been left exposed to bees and humidity in an experimental hive for over a year, and had then ridden on the floorboards of my truck for some hot summer months — allowing plenty of time and exposure for the amitraz to degrade.
So I had everything I needed to run a simple experiment to determine whether (1) actual contact was necessary to quickly kill mites, and (2) whether the amitraz in the “aged” strips had degraded to a measurable extent. As it turned out, my initial results were so interesting, that I expanded the experiment. In writing up the results, I thought that it would be of interest to beekeepers to see just how I ran some controlled experiments (meaning that I ran both Test and Control groups), with multiple replications (meaning repeating the experiment again and again [[4]]).
Preparation
I first ran a few preliminary tests to see how many bees I should put into each cup, and how best to modify the cups so that I could accurately collect any fallen mites over time. And then I was fortuitously visited by Dr. Jeff Pettis, who shared some tips as to how he was preparing “Pettis Test” cages for an amitraz-resistance project that he was undertaking. This information helped me to zero in on how to use measured pieces of Apivar strip to apply consistent doses of amitraz that were not too weak, or too strong.
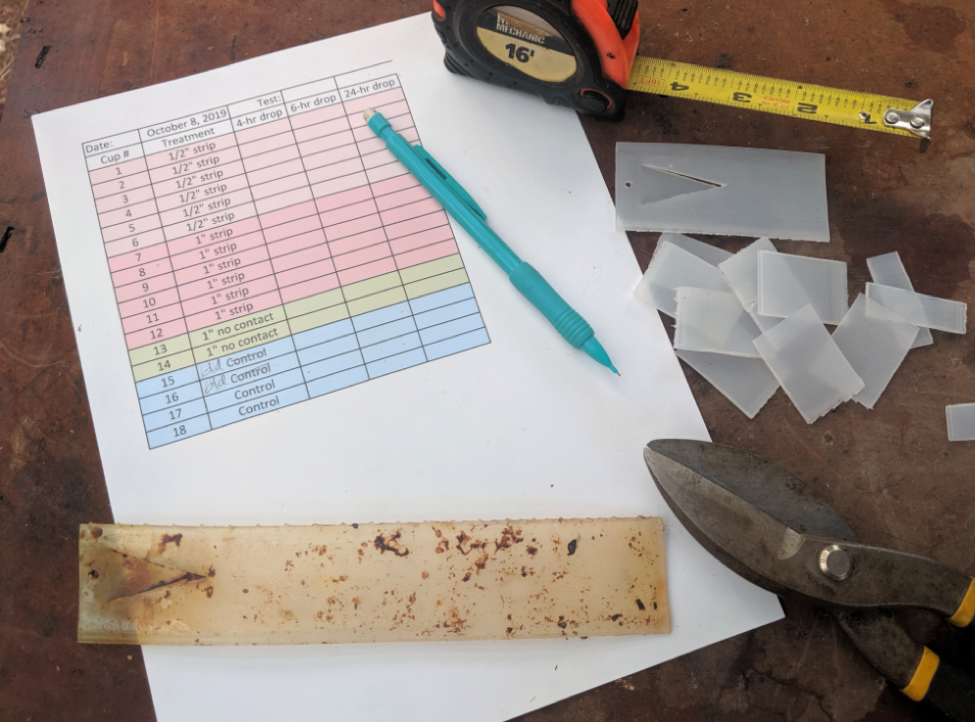
I then prepared color-coded data sheets, and fabricated a bunch of fresh cup cages — each clearly numbered. I cut freshly-opened Apivar strips (stored in a freezer) into carefully-measured cross strips, doing the same for aged strips (Fig. 1).
Figure 1. I used shears to cut Apivar strips crossways into ½” or 1” pieces, in order to apply consistent dosages to the bees. The well-aged strip in the foreground is about to be cut up.
I experimented with various locations in the cages to place the strips, observing the behavior of the caged bees, in order to see where they most often rubbed against or walked over the strips. I decided upon hanging the strips from screened lids, since when exposed to light from above, the bees would crowd to the top of the cage, and walk back and forth over and against the strips. The cup cages consisted of a modification of my mite wash cups — an inner cup with a screened bottom (through which mites could drop), inserted into an outer cup with a screened lid. This design would not only allow easy counting of any dropped mites, but would also allow me to easily perform an alcohol wash (in the same cups) to recover any mites remaining on the bees after the 24-hr treatments. I wore fresh nitrile gloves to avoid unintentional contamination of any cup, preparing the Control group cups first (Fig. 2).
Figure 2. Thanks to a tip from Dr. Jeff Pettis, I used bobby pins to suspend the measured pieces of Apivar® strips at the top of the cages. You can just make out the pieces of Apivar strip lying flat at the bottoms of two cups in the center row, separated by a 1 3/8” air gap below the lower screen – preventing any possible direct contact with the strip by the bees, yet ensuring that any vapors would need to pass up around the caged bees.
The test groups that I ran were:
- Controls (no Apivar strip, to determine a “baseline” value for the percentage of mites expected to drop without treatment)
- A ½” hung strip
- A 1″ hung strip
- A 1″ used/aged hung strip
- A 1″ strip placed below a screen, no contact
I then took the prepared cups out next to high-mite hives, shook bees into a plastic tub, and used a 30 mL cup to scoop up samples of ~100 bees for each cage [[5]] (Fig. 3).
Figure 3. Here I’m dumping 30 mL of shook bees into a cup. The cups, due to their heavy screened lids, were tippy, so I temporarily snapped plastic lids over them for transport to the incubator. I stocked the cups with bees in the morning, so that I could take 4- and 6-hour mite drop counts later in the day.
Once in the incubator, I placed a feeder of 1:1 sugar syrup over each cup [[6]], and turned on an overhead light for several hours to encourage good bee contact with the strips during the day (Fig 4).
Figure 4. Here I’m placing tubes of syrup on the test cups in my home-made incubator, with the light on. At night I turned the light off, so as to minimally interfere with the bees’ accustomed photoperiod. It is critically important not to stress or agitate the bees unnecessarily, since that could add a variable that might cause their mites to drop off.
In all, I ran six replicate tests during October and November, stocking over 100 test cups in total (Fig. 5).
Figure 5. The incubator maintained temperature within a degree of 30 C (86 F), and around 40% relative humidity. There was scant bee mortality.
I hadn’t initially planned to run so many replicates, but with each run, as I observed the effects of the various treatments, and to ensure that I had run enough repeats of each treatment to notice any trends, I adjusted the number of cups in each test group in subsequent runs [[7]]. Of interest, I ran two additional cups with a full Apivar strip wrapped around the inside, just to see what would happen. That high an exposure greatly agitated the bees, and caused them to consume far more syrup than any other test group. By 24 hrs, the full strip killed all the mites, but there was no substantial increase in bee mortality.
On my first run, I enjoyed a visit from Arkansas State Extension Apiculturist Dr. Jon Zawislak, who was speaking to our local club (Fig. 6).
Figure 6. Dr. Zawislak helped me to process the first run. Two mechanical agitators are in the foreground, which provide consistent and complete recovery of any mites still on the bees at the end of each experiment. I greatly enjoy bouncing ideas off other researchers, seeking challenge and constructive criticism.
Below is another view of my state-of-the-art covered outdoor lab, which we normally use for our cell starter colonies in springtime. I didn’t think to clean up before Jon snapped some photos (Fig. 7).
Figure 7. I cleared a spot on the multipurpose “lab bench” to process the samples. Here I’m pouring alcohol into a cup for a final mite recovery. Behind me are mite-free test hives with stickyboards, which I monitor twice a week to determine the rate of mite immigration taking place in this yard (independent of this experiment).
At 4, 6, and 24 hours (depending upon the run), I removed the lower cup and counted any fallen mites (alive or dead), wiped it clean, and replaced it. After counting fallen mites at 24 hours, I then added alcohol to cover the bees, and ran the cup for two agitations on my agitators, which I can count upon to obtain 99%+ recovery of any mites yet remaining on the bees (Fig. 8).
Figure 8. It’s easy to assume complete mite recovery, but I confirm by test. I saved the washed bees in a tub and then rewashed them to make sure that I was actually obtaining 99%+ recovery.
Between runs, if I changed the treatment type in the numbered cup cages, I would discard any cup that had come into direct contact with an Apivar strip. The rest of the cups and screened lids I washed in hot detergent water, and then rinsed in acetone to eliminate any residues of treatment.
The results
This experiment was all about the comparative differences in mite drop between the various treatments, including the untreated Controls. Surprisingly, there was a goodly amount of variation in the percentage of mites that dropped in the controls over 24 hrs.
Practical application: Due to the inherent variability of most any measurement involving honey bees or mites, one needs to run a number of repeats and, preferentially replicates — running the entire test again and again. Of concern is that I find that some of what we think we know about bees and mites is based upon the findings of a single study, not yet replicated by others.
I graphed out the results below, choosing to show means, which I felt represented the data better than median values (since for both the ½” and 1” Apivar treatments, median efficacy at 24 hours was 100%). As usual, I typically graph the results of an experiment in several ways, to try to make them as easy to digest as possible. In this case, I decided that the best way to present the data was to show what percentage of the original mites remained on the bees at each time point (Fig. 9).
Figure 9. Most of the mites stayed on the bees in the untreated Controls (red line). To my surprise, there appeared to be some degree of vapor action from the no-contact piece of Apivar strip (orange line). Exposure of the bees to either a ½“ or 1” strip of Apivar (blue lines) dropped most of the mites within 4 hrs, and nearly all by 24 hrs. Again surprisingly, the “aged” Apivar strip (black line) still performed pretty danged well.
When comparing the effects of the treatments, keep in mind the “background” drop of mites in the Control group. In half of the 26 Control repeats, no mites dropped at all; but in a few as much as 44% dropped, which skewed the mean. Overall, there was a 10-12% (mean or median, respectively) drop of mites in the Control cups at 24 hrs independent of any treatment. By comparison, there was 100% mite drop by 24 hrs in three-quarters of all cups with any type or amount of hung strip.
Practical application: In one of the replicate runs, due to using bees from a colony with a lower infestation rate, I doubled the number of bees in the cup (to ~200). The two highest mite drop rates for Control cups (40 & 44%) occurred in this run. This observation suggests that the number of bees to use in a cup should be further investigated for this sort of testing.
Time certainly appeared to be an important element of exposure for the ½“ or “no contact” treatments, which took longer than 4 hours to exhibit full effect.
Discussion
It looks as though I can now answer my two questions:
Q1: Does amitraz only work by direct contact? The results certainly suggest that despite amitraz’s “negligent” vapor pressure, that even having a full 1 3/8” separation between a 1” strip (laid on its side, so no evaporation from that side) did not prevent the strip from dropping over 40% of the mites, after subtracting for “natural” mortality. So yes to vapor action even at below broodnest temperature.
FWIW, back in the day, vaporization of amitraz by various heating methods was commonly employed. This is extremely dangerous for the operator, and I’ve met a beekeeper who nearly lost his life from doing it. However, it is of great interest that mites could be affected by vapors being emitted from an Apivar strip. This subject certainly calls for more research.
Q2: Does amitraz degrade rapidly? Despite the warnings to use the strips quickly after opening, these limited experimental results suggest that the active ingredient continues to emanate from the plastic strips for quite some time.
Acknowledgements
Thanks to Drs. Jeff Pettis and Jon Zawislak for their assistance.
References
[1] https://www.dadant.com/wp-content/uploads/2012/04/2011/09/FAQs_Apivar_US_2012.pdf
[2] Williams, G, et al (2013) Standard methods for maintaining adult Apis mellifera in cages under in-vitro laboratory conditions. Journal of Apicultural Research 52(1): 1-36.
[3] It’s difficult to obtain meaningful data on the effect of anything upon varroa riding on bees unless mite counts are high – at low mite infestation rates, it’s just difficult to separate any “signal” from the “noise” of random variablility.
[4] As opposed to the error of “pseudoreplication” – counting each test cup in the same run as a replicate.
[5] In one replicate, due to a lower mite load, I used 2 scoops per cup.
[6] Which I found to be necessary for any test over 4 hours.
[7] Ideally, I would have run exactly the same number of test groups and repeats in each replicate, but this started out to be a “quick and dirty” experiment that grew with interest each replicate.