Re-Evaluating Varroa Monitoring: Part 1 – Methods
Re-Evaluating Varroa Monitoring
Part 1
METHODS
Randy Oliver
ScientificBeekeeping.com
First published in ABJ March 2020
In order to avoid the preventable death of colonies due to varroa-virus overload, we’re told to monitor our colonies’ mite levels. But how best to do so? In this series of articles, I’ll review what we actually know, and provide hard data from my own recent research to improve our methodology.
Most successful beekeepers (as measured by having high rates of colony survival) monitor mite levels in their hives to some extent. There are a number of methods to do so, each with its pros and cons. Let me start off with a brief review of different ways used to monitor varroa levels, and then I’ll move onto recent studies that I’ve performed in order to answer some questions that I had.
Whole-hive Assessment
- Visual inspection. One might think that you could simply look for mites on the bees or for bees with deformed wings. Unfortunately, due to the fact that most mites may be sequestered in the brood, and those on the bees hidden on their undersides, by the time these signs become apparent, it’s already too late, since the in-hive DWV explosion has passed the tip point.
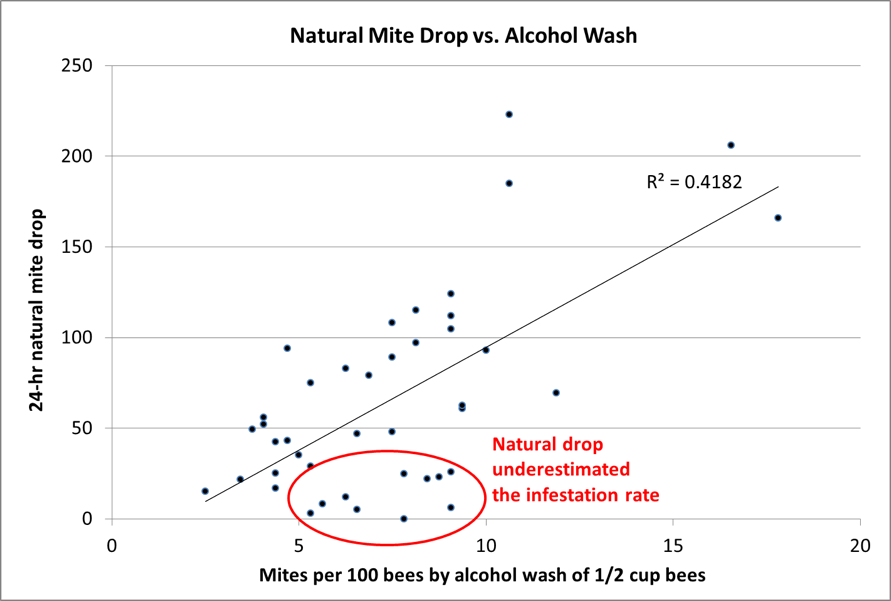
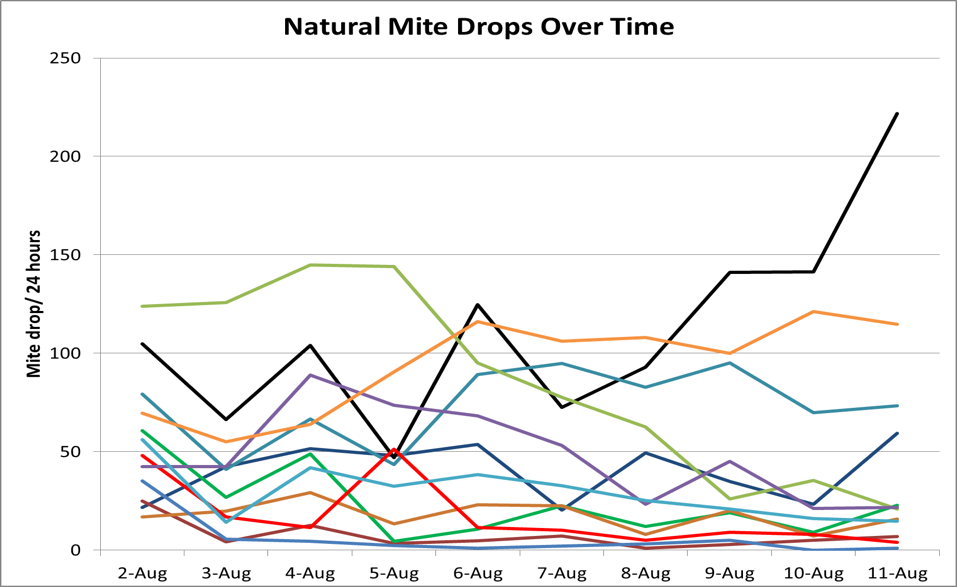
- Natural mite fall, aka stickyboard counts. This method is popular since it does not require the opening of the hive or handling of bees. The results of field comparisons of natural mite drop to the total colony population of mites are mixed, with some researchers finding a good correlation [[1]], and others not so much [[2]]. My own data [[3]] suggest that natural mite fall can greatly under-or overestimate the actual infestation rate of the bees in the hive (Fig. 1), and vary widely day to day for the same hive (Fig. 2).
Figure 1. Natural mite fall counts in late July and early August, compared to alcohol washes taken at the same times. Note how stickyboard counts can greatly underestimate a serious mite infestation.
Figure 2. Daily natural mite drop for a dozen hives that I monitored in an experiment. Note that the daily drop can vary substantially, since it has much to do with the amount of brood emerging each day.
Dr. Richard Fell [[4]] also tracked natural mite drop over time, and concluded that:
The results of this study… indicate that mite-sampling data can be highly variable. Mite numbers from sticky board samples were found to vary by as much as 250% in as little as two weeks.
Natural mite drop is strongly affected by high death rate of mites immediately after emerging from a cell, resulting in bands of mites on the stickyboard directly below frames [[5]]. Natural mite drop counts must be also be assessed relative to each colony’s strength, since 25 mites falling from a 4-frame colony is more serious than 25 falling from a 20-framer. The method does require sharp eyes to pick out the mites from the hive trash, is tedious, and requires a return trip to the apiary.
- “Forced” mite drop. One can force a more rapid mite drop, which better reflects the infestation rate of the adult bees, by applying a treatment to cause the mites to release their grip, such as oxalic drip or vapor, powdered sugar, formic acid, or a fast-acting synthetic miticide. A forced drop can give relatively quick and fairly accurate results. There are several advantages to this method:
- No need to remove any frames (Fig.3).
- No harm to the bees or queen.
- Dusting takes only a few minutes.
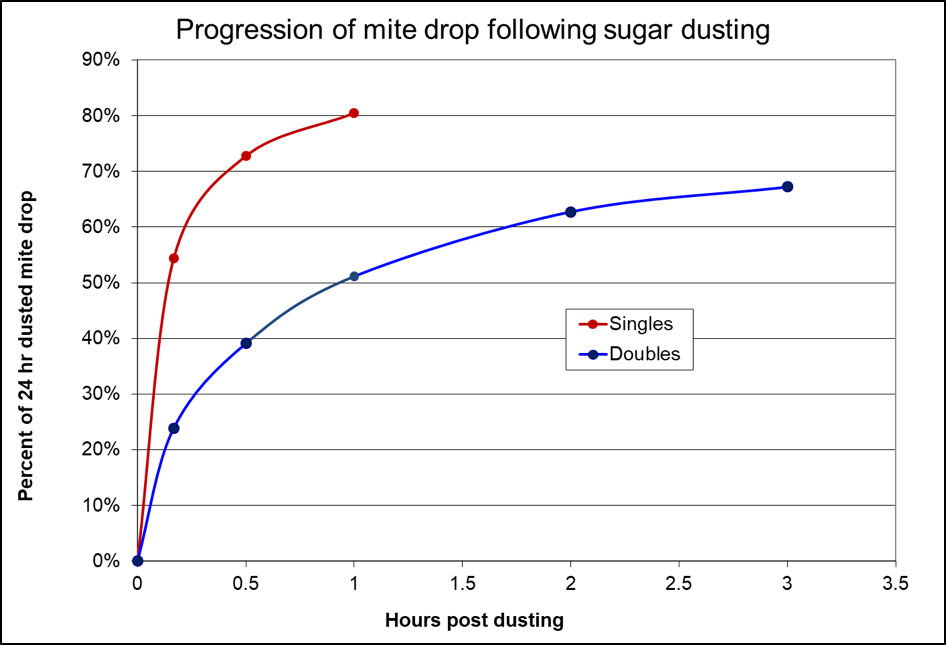
- Mite count in less than an hour (Fig. 4).
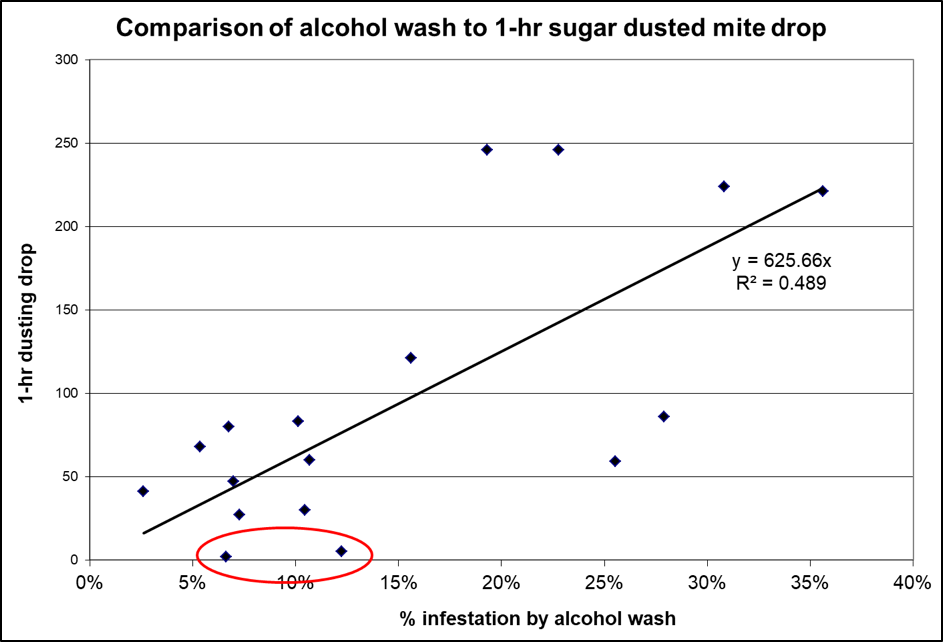
- The mite drop is fairly representative of the overall infestation rate (Fig. 5).
- The treatment kills a proportion of the mites on the bees, so you kill two birds with one stone.
Figure 3. In this old photo we were applying powdered sugar to force a mite drop [[6]]. A forced drop is a quick and easy way to get an idea of the mite infestation rate of the adult bees.
Figure 4. Powdered sugar causes the mites to drop rapidly in a single deep hive, but take a bit longer when dusted only over the top bars of a double. In my tests, dusting causes about a third of the phoretic mites to drop.
Figure 5. As with natural mite fall, an accelerated drop must be adjusted for the number of frames of bees actually dusted. The above data is from double deep hives. Note, however, that for two of the hives with appreciable mite infestation rates (circled), that almost no mites dropped in the first hour (nor had many dropped even by three hours, not shown).
Brood dissection
- Typically drone brood dissection: This tedious method can quantify the percent of brood cells infested by mites, but which 100 cells to sample? As pointed out by Fuchs [[7]], the distribution of mites in brood cells varies greatly from comb to comb, and within the cells of a patch of brood (Fig. 6). Charriere [[8]] found that “The parasite load of drone cells was seen to vary from one- to six- times in the space of a week, without any relation to the actual varroa population”. In our operation, we run a drone comb in each hive, but after inspecting many frames of drone brood with a cappings fork, I concur with Charriere. And in in dry climates, there is little drone brood available for inspection during the critical late-summer period anyway.
Figure 6. It’s definitely worthwhile to quickly inspect broken-open drone brood on the top bars. We do so as we are splitting our colonies after almonds – if we see mites in the drone brood at that time we take action. But as far as being a reliable monitoring method (even with a drone trap frame), it is inconsistent.
Practical application: According to BIP surveys [[9]], beekeepers use mite drop and drone brood inspection about as much as they do the adult bee sampling methods below. Mite drop count gives a general idea of the total mite population in a colony, but must be interpreted relative to the amount of bees and brood in the hive. Not so with the methods below, for which a good case to be made that determining the infestation rate of the adult bees is more biologically relevant to colony health.
Adult bee sampling
All the methods below involve taking a sample of a half cup of workers (~315 bees). But since these bee samples represent fewer than 1% of the bees in the colony, the choice of comb is important (to be covered later), as well as how the bees are handled prior to being placed in the jar, how accurately the half cup is leveled off, and how the mites are then dislodged and recovered from the bees.
Practical note for baby boomers: any method that involves identifying and counting mites requires good close-up vision. Put your danged reading glasses on!
I’ve listed some commonly-used methods below, in order of expected mite recovery. However, that recovery is all in the details, which I’ll investigate in a following article.
- Alcohol wash. I’ll unabashedly state that this is my favorite. I originally used Dr. Medhat Nasr’s handy design for a shaker made from two plastic peanut butter jars [[10]]. But a problem with the two-jar method is that a percentage of the mites get caught in the bees after the last shake, as the alcohol filters down through them [[11]]. I found that the method can be improved by instead using two nested cups and using a swirling action – as opposed to shaking — to avoid washing the mites back up into the bees with each shake. Instructions for such cups are at [[12]].
- Powdered sugar roll. Can be accurate if properly performed – by rolling the bees in the sugar prior to shaking, which causes them to heat up and make their mites release. The required full minute of vigorous shaking is hard on the arms – use a lightweight plastic jar. The main advantage of this method is that the 300 bees are only brutalized rather than sacrificed
- Detergent wash. This method also works quite well, especially if performed with non-sudsing powdered dishwasher detergent.
- Ether roll. Quick, reasonably accurate [[13],[14]], and widely used, but it may exhibit poor mite recovery [[15]].
- CO2 Although very attractive since the CO2 doesn’t kill the bees, I found the method to be inconsistent [[16]].
Practical note: all of the above methods are only as good as how well you perform them. If you cut corners, or don’t exactly understand the importance of details in technique, your mite counts may not be reliable.
I’ve found the alcohol wash to be the most reliable method, as did Azizi [[17]]. It’s quick, consistent, and can result in better than 95% mite recovery in a minute, with the sugar roll a close runner up. In our selective breeding program, we are now performing over 2000 alcohol washes per year. We graduated from hand agitation of the wash cups to machine agitation in the field. We now use portable mite wash agitators of my own design, with 60-second built-in timers, calibrated to recover at least 95% of the mites in the first minute (I’ve got a 1-minute video at [[18]]). We now fly through our apiaries taking bee samples (Fig. 7).
Figure 7. Our selective breeding program for varroa resistance is based upon alcohol wash counts. It occurred to me that it was time to confirm the reliability and details of the techniques that we were using.
I realized that our methods were largely based upon interpretations and assumptions rather than hard data. I couldn’t truly say that I knew the answers to the following questions:
- Does the mite infestation rate of sampled bees vary much from comb to comb in a hive?
- Which combs in the hive produce bee samples that best reflect the infestation rate of the adult bee population of the colony as a whole?
- Is it necessary to disrupt the broodnest by pulling brood combs, and can we minimize the chance of inadvertently killing the queen by avoiding the broodnest?
- Does allowing some shaken bees to fly out of the tub prior to scooping up a sample, affect the mite infestation rate of the remaining bees?
- Does it make a difference which concentration of alcohol that we use in an alcohol wash?
- How strongly do we need to agitate the bees to dislodge the mites?
- Which agitation action is the best to apply during the wash?
- Does it make a difference how long we agitate the bee sample in the alcohol?
- What sort of mite recovery can we expect to obtain? Can we consistently get 95%+ recovery? Can we get 100%?
Yeah, a lot of good questions – the first half of them apply to adult bee sampling in general, the second half specific to alcohol wash. Yet despite the alcohol wash being so widely recommended, it’s hard to find hard data to answer those questions.
The best data is obtained from colonies with high mite counts, otherwise it’s difficult to separate out the effect of any detail from random variation. So when I had a number of high-mite hives at my disposal late last season, I ran a ton of tests to answer the questions above. Some of the answers surprised me.
Coming: In my upcoming articles, I’ll present data to help answer each of the above questions in turn.
References
[1] Delaplane, K & W Hood (1999) Economic threshold for Varroa jacobsoni Oud. in the southeastern USA. Apidologie 30: 383-395.
Bieńkowska, M & Konopacka (2001) Assessment of honeybee colonies infestation by the mite Varroa destruktor based on its natural mortality during the summer season. Vol. 45 2001 Journal of Apicultural Science 45: 129-140.
[2] Rademacher E. (1985) Ist eine Befallsprognose aus dem natürlichen Totenfall von Varroa jacobsoni möglich? Apidologie, 16(4): 395-406
[3] https://scientificbeekeeping.com/mite-management-update-2013/
[4] Fell, RC, et al (2010) The spatial distribution of varroa mites in honey bee hives. Proceedings of the 2010 American Bee Research Conference
[5] Ibid.
[6] https://scientificbeekeeping.com/fighting-varroa-biotechnical-tactics-ii/
[7] Fuchs, S (1985) Quantitative diagnosis of the infestation of bee hives by Varroa jacobsoni Oud. and distribution of the parasitic mite within the hives. Apidologie 16(4): 343-368.
[8] Charriere, JD, et al (2003) The removal of capped drone brood: an effective means of reducing the infestation of varroa in honey bee colonies. Bee World 84 (3): 117-124.
[9] https://research.beeinformed.org/survey/
[10] Nasr, M & A Williamsongg (2010) Varroa hand shaker: a simple field method for monitoring varroa mite infestations. Proceedings of the 2010 American Bee Research Conference.
[11] See Table 1 at https://scientificbeekeeping.com/mite-management-update-2013/
[12] https://scientificbeekeeping.com/an-improved-but-not-yet-perfect-varroa-mite-washer/
Also https://scientificbeekeeping.com/mite-washer-still-improving/
[13] Devlin, S (2001) Fig. 7 in Comparative analyses of sampling methods for varroa mites (Varroa destructor Anderson and Trueman) on honey bees (Apis mellifera L .) M.S. Thesis, Simon Fraser University.
[14] Rinderer, T, et al (1990) Varroa mite detection in beehives: evaluation of sampling methods using tobacco smoke, fluvalinate smoke, amitraz smoke and ether-roll. American Bee Journal 130(2): 127-129.
[15] Azizi,H, et al ( 2008) The comparative evaluation of the laboratory methods of separation mite varroa from the mature honeybee. Research Journal of Parasitology 3: 123-129.
[16] https://scientificbeekeeping.com/a-test-of-using-co2-for-bee-friendly-mite-monitoring/
[17] Azizi,H, et al ( 2008) Op cit.
[18] https://www.youtube.com/watch?v=p8ckGYwCJ7U