The Nosema Problem: Part 5 – Monitoring and Disinfection
Contents
The Nosema Problem: Part 5
Monitoring and Disinfection
First published in ABJ October 2019
Randy Oliver
ScientificBeekeeping.com
Some beekeepers are concerned about the current unavailability of fumagillin. Although the Canadians are working to get it back on the market, there remains the question as to just how important it actually is to apply a prophylactic treatment to suppress nosema.
Back in the day, I followed the commonly-accepted practice of applying prophylactic treatments of Terramycin® to my hives in order to prevent AFB, Fumidil B® for nosema, grease patties for tracheal mite, and then Apistan® to every hive for varroa. But as our operation grew, and as we learned what was really necessary for colony health, we now rarely use Terramycin, no grease patties, only “natural” treatments for varroa as needed, and no “hive health” products. Yet our colonies thrive with minimal losses, and we sell 1000 healthy nucs every spring. This change in management was not for idealistic reasons, but rather based upon business decisions as to what gave us the best returns on investment.
That said, our “new” nosema still remains something of an enigma, and I’m not about to make any suggestions as to how you “should” best manage it in your apiaries. But I’ve now had over a decade of experience with tracking nosema in my own operation, as well as following research from around the world. I’m happy to share my confusion with you.
What the beekeeper can do
The first question that I ask beekeepers who approach me about a nosema issue is, “Have you checked with a ‘scope to confirm that nosema is actually a problem in your hives?” The usual answer is “No, but I saw dysentery.” I hope by now that I’ve made clear that nosema does not appear to cause dysentery, but that dysentery in the hive will likely increase transmission of the parasite (if it is indeed present) within that hive.
Practical application: A brief bout of dysentery in the spring does not mean that the colony is infected with nosema. However, if you observe dysentery, you may be able to put your mind at ease by checking some bees under a ‘scope. On the other hand, if you observe a colony that is inexplicably not exhibiting a normal rate of buildup in the springtime, an assessment for nosema would be wise.
seasonality
The first thing to keep in mind is that nosema, whether apis or ceranae, is mainly an autumn or springtime issue, and typically causes serious disease only when the workers are constrained by weather from being able to take defecation flights. The seasonality of ceranae was elucidated clear back in 2008, when Spanish researcher Dr. Mariano Higes [[1]] published illustrative charts of the progression of N. ceranae prevalence in the house and field bees over the course of two years. Since then, other researchers have confirmed that ceranae infection tends to follow the same track as that of apis. However, ceranae, unlike N. apis, may sometimes be found during summer.
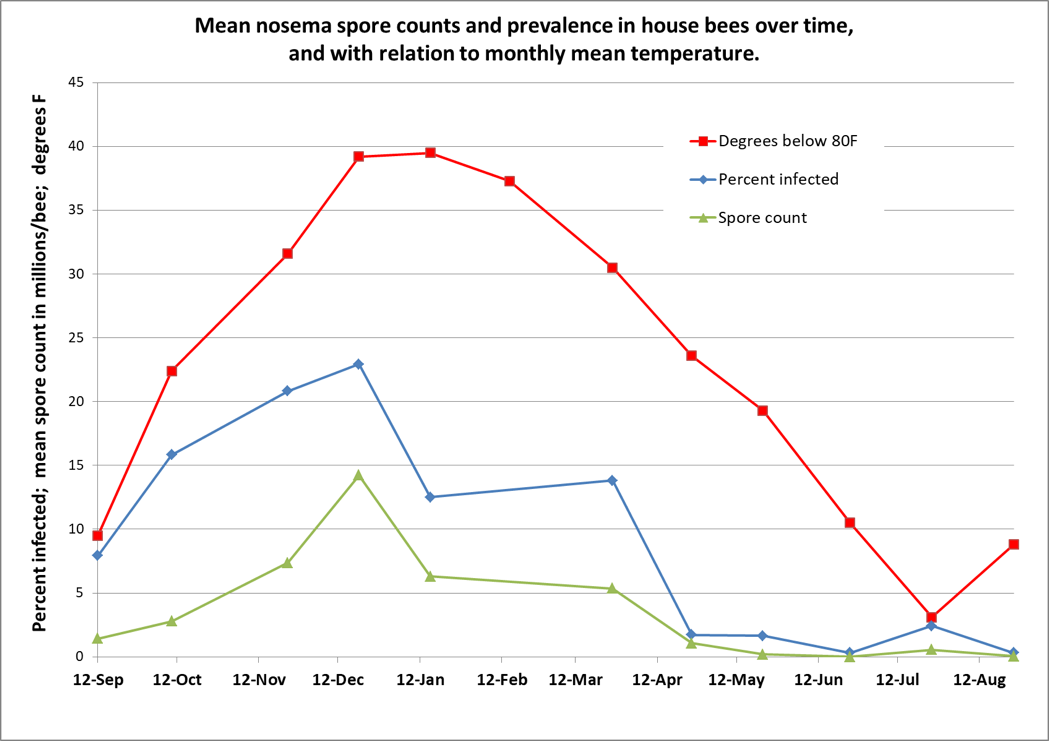
In 2012, Chen [[2]], in Taiwan, found that the infection intensity of N. ceranae over the course of a year closely correlated with the local ambient temperature ― with greater infection when it was colder, then disappearing during the warm days from July through September. This finding surprised me, since Traver [[3]] had reported the year before that ceranae was prevalent in Virginia during the summer. So I questioned Chen at length to confirm his methodology, and then, in one of my own apiaries in the California foothills, tracked N. ceranae spore counts and prevalence (the percentage of house bees infected) over time [[4]]: It was the same in California ― nosema infection was clearly related to temperature (Fig. 1).
Figure 1. I tracked nosema spore counts and prevalence in an apiary for a year. I started with 36 colonies, and ended with 31, but there was no apparent correlation between colony survival and nosema levels. However, there was a clear relationship between nosema and the average monthly temperature (which I flipped upside down to show the correlation). In my area, colonies enjoy their first pollen flow in early January, and then build rapidly, largely clearing themselves of the parasite by May. Not shown is that the median value for percent of house bees infected never exceeded 20%, although one sample had 80% of the bees infected in December (surprisingly, that colony eventually completely purged its infection without treatment).
Practical application: Seasonally speaking, there’s likely little reason to be concerned about nosema other than during pollen flows in the autumn or spring, and only then if there is poor flight weather.
So that now brings us to the subject of how best to determine the level of infection by nosema in the hive.
detection and Sampling
I’ve written about this subject previously [[5]]. What most surprised me, after being scared to death about the invasion by N. ceranae, is that after I went back in time to read the open-access deep dive into N. apis by GF White, written in 1919 [[6]], that it appears to me that there is little difference between the two species as far as impact upon the colony. Allow me to quote directly:
The prognosis in Nosema-disease varies markedly and is dependent upon the conditions present. Of these conditions the percentage of Nosema-infected bees in the colony, the strength of the colony, the season of the year, and the environment of the apiary are among the most important factors which determine the outcome of the disease….As a rule colonies which in spring of the year show less than 10 per cent of Nosema-infected bees gain in strength and the losses are not detected…When the number of infected bees approaches 50 per cent the colonies become noticeably weakened and in many instances death takes place…As a rule, the stronger the colony, the more favorable is the prognosis.
Everything that I’ve observed or read agrees with what White wrote back then. So the question then is, how to determine what percentage of the bees are infected? White’s method of dissecting individual bees was tedious, so a shortcut was later developed by others to simply grind up 10 bees and count the number of spores in a hemocytometer to obtain an “average” spore count. Thus, we got hooked on “spore counts,” and when people (including myself) started taking spore counts from bees taken from the entrances of hives, those counts often left us gasping in alarm.
Practical application: I’ve given up on spore counts as being very meaningful. What I’ve also learned over the years is not to sample bees from the entrance, as it will just scare you, and doesn’t tell you what’s happening inside the hive.
Many researchers now sample “house bees,” typically taken from under the lid or from an outside frame. This gives you a “more accurate and representative sample of the whole bee population” of the hive [[7]]. And even then, spore counts can be misleading. Retschnig [[8]] points out that if you happen to sample a bee ready to take a defecation flight, that it will skew the spore count upwards (due to all the spores passively sitting in its hindgut).
Mulholland [[9]], using molecular analysis, found only a weak correlation between the degree of infection of a bee’s midgut tissue (where nosema replicates) and the number of spores counted. He also noted that:
Composite samples with only one or a few highly infected individuals can present a skewed infection level for a colony. For example, a few heavily infected bees in a composite sample could generate the same spore count as a sample with many moderately infected bees.
My point is, that I don’t bother any more with spore counts. Instead, I first determine whether any bees in the hive are infected by doing a quick crush of 10-25 bees in a sandwich bag [[10]]. Only if spores are clearly present do I then move to the next step and check for prevalence ― the percentage of infected house bees (rather than the intensity of infection of those bees) [[11]]. N. ceranae, at least, makes this easy, since in general, most any bee is clearly “infected” or “uninfected.”
Practical application: Although in my graph above, spore counts roughly tracked prevalence, that is not always the case. What I observe time and again is that the spore count from an entrance sample may scare you, but the prevalence of infection of the house bees from the same hive may be quite low [[12]].
Cameron Jack and collaborators published an enlightening study in 2016 [[13]], and concluded that:
These results further indicate the risk of using spore counts from composite samples (samples containing multiple bees) for making inferences regarding N. ceranae infection in colonies… Hence, it appears that analysis of individual bee samples would be ideal to accurately diagnose a colony’s infection level even though it is more time-consuming and expensive…
Practical application: I’ll be the first to admit that performing individual gut squashes of bees takes a little effort, but it actually isn’t difficult (and I’d rather do it than hemocytometer counts). Using disposable cover slips, I can personally check 10 bees for the presence of infection in less than 5 minutes. Five minutes of my time at the table with a ‘scope is well worth saving the cost of unnecessary treatments, and sure keeps me from wondering whether nosema is actually an issue in my hives. You’ll also (if you review the step-by-step photos at [[14]]) be able to diagnose if there is something else wrong with the bees’ guts ― especially if you are observing dysentery. I’ve personally found it to be quite illuminating.
the biological relevance of Prevalence vs. intensity
An important difference: “prevalence” indicates the proportion of the sampled bees found to be infected – this is what is biologically relevant to colony health, since nosema generally isn’t a problem to a colony until at least 20% of the house bees are infected (2 infected bees out of 10 in a sample). “Intensity,”on the other hand, only indicates the average number of spores per bee in a sample (in millions). It is thus only a very rough proxy for the proportion of bees actually suffering from disease, and, especially in the case of N. ceranae, can often misrepresent the degree of infection of the colony as a whole.
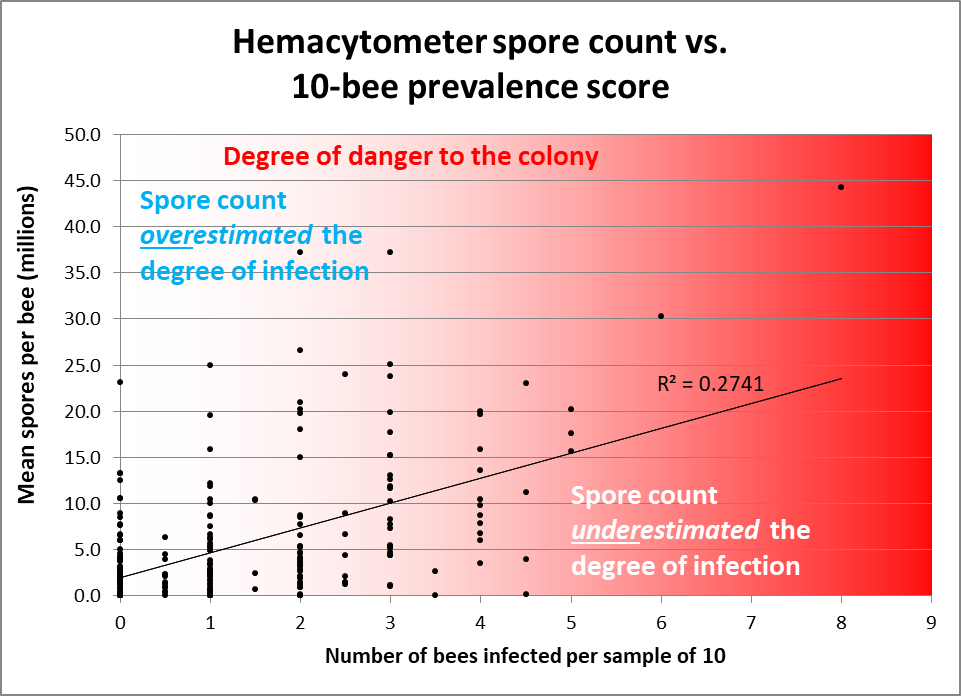
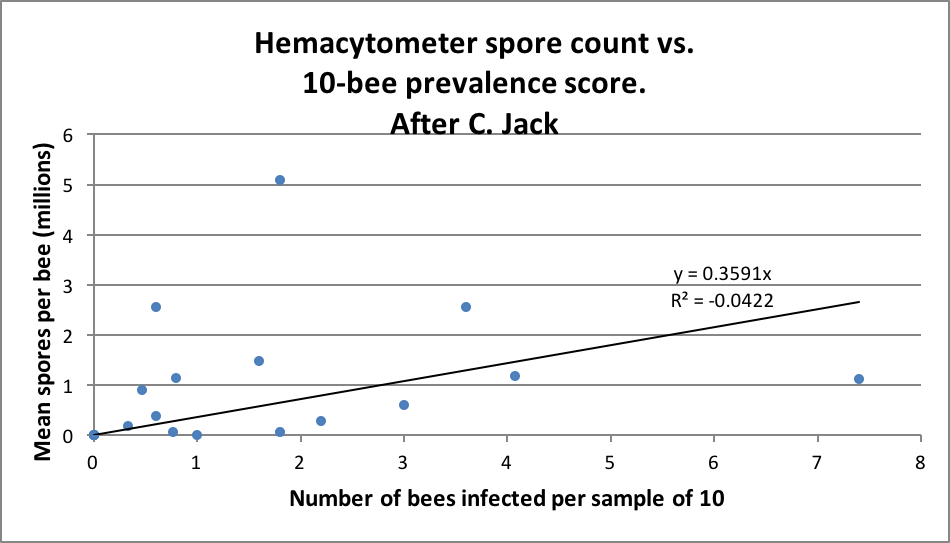
The above said, let’s compare some spore counts to prevalence counts for samples taken and analyzed both ways ― the first graph is of my data; in the second I plotted out Jack’s data (Figs. 2 & 3).
Figure 2. Note how often spore counts miss the mark as to the actual percentage of bees in the hive infected, and the very weak overall correlation (data points from divided samples of house bees — 10 individually inspected, 25 homogenized for a spore count [[15]]). I shaded with red in order to indicate the actual degree of danger to the colony. Colonies having 2 or fewer infected bees out of 10 would likely be nothing to worry about, but often exhibited spore counts in the tens of millions. Conversely, spore counts often underestimated the degree of prevalence of infection in hives having 30-50% of the bees infected.
Figure 3. Jack’s data (from his thesis) largely reflect mine ― spore counts often overestimated the degree of infection, and sometimes seriously underestimated the same. Note that there was a much lower rate of intensity of infection overall in Jack’s samples than in mine above.
It’s worthwhile to read Jack’s entire thesis [[16]], in which he suggests:
My results demonstrate that optimal pollen nutrition increases Nosema ceranae intensity, but also enhances the survival or longevity of honey bees. The information from this study could be potentially used by beekeepers to formulate appropriate protein feeding regimens for their colonies to mitigate Nosema ceranae problems.
Practical application: Go into winter with strong colonies, feeding high-quality pollen sub if necessary. Sample some for nosema prevalence before giving perhaps unnecessary treatments. Check for nosema prevalence again in springtime to either assuage your fears, or to confirm that treatment is indeed indicated.
Queens And Nosema
Running young queens in one’s hives is always a good idea, due to their typically more vigorous egg laying and pheromone production. Those attributes also appear to help colonies to fight nosema. Botías [[17]] ran an experiment to see how replacing the queen affected N. ceranae levels in colonies in Spain:
Indeed, the removal of the queen and the subsequent replacement with a younger queen decreased the proportion of Nosema-infected forager and house bees, which maintained the overall infection at a level compatible with colony viability.
The above findings were confirmed by Simeunovic [[18]], who also indicated that older queens may be infected by N. ceranae.
Practical application: Colonies with young queens may be able to handle N. ceranae better than those with older queens.
A common question raised by beekeepers is whether nosema is causing the premature supersedure of purchased queens. Furgala [[19]] found most, but not all, queens to be highly susceptible to Nosema apis. It’s not so clear that queens have as much problem with N. ceranae. I’ve checked a number (as did Cameron Jack and Botías), but none of us found an infected queen.
Deadout equipment
Dr. White [[20]] found that dysentery was not necessary for the transmission of N. apis, and Smith [[21]] determined the same for N. ceranae. But if you’ve ever watched a bee defecate within an observation hive, it’s clear that the mess is quickly consumed by other bees — a sure way to infect that bee. But when I’ve inspected combs from colonies laden with N. ceranae, I couldn’t detect any spores on them [[22]]. But another researcher sent me a comb that did have spores on its surface, so I’m not clear on comb contamination.
White performed an experiment to determine whether Nosema apis could be transmitted via infected brood combs from heavily-infected colonies, by inserting them into nosema-free healthy hives. He did so with 14 hives, inserting the infected frames at various times from April through July. None of the hives got infected. Note, however, the date range in which he inserted them — during the time period in which colonies can typically purge the parasite.
Of interest is when I first became aware of N. ceranae, I sent samples of spores to mycologist Dr. Rob Cramer at the University of Montana. We were greatly surprised that most of the spores became nonviable after an overnight refrigeration. I brought this observation up with the late Dr. Ingemar Fries, who then ran an experiment that found that either refrigeration or freezing appeared to greatly reduce the viability of the spores of N. ceranae [[23]].
But it wasn’t quite that simple. One of Dr. Steve Pernal’s students, Courtney MacInnis, measured the viability of N. ceranae spores stored under different conditions [[24]]:
Practical application: Most spores on beeswax died within a few weeks at broodnest temperature or if frozen; but at room temperature some survived for up to a year. Surprisingly, although freezing quickly kills spores on beeswax, it helps them to survive longer if they are immersed in honey, in which they can survive frozen for well over a year. If the honey, on the other hand is kept at broodnest temperature, the spores only remain infective for several months. So either freeze your contaminated combs or keep them warm, but don’t feed back honey that’s been stored frozen.
Combs without honey can also be decontaminated by fumigation with acetic acid (I’m curious as to whether formic might also do the trick). And Dr. Frank Eischen found that they could be disinfected with Phostoxin [[25]]. The spores are also easily killed with bleach, but I don’t have hard data on the best application method.
Practical application: In our operation, we routinely reuse the boxes of combs from deadouts without performing any disinfection whatsoever (other than to check for AFB). But we typically reuse those combs later in the springtime when colonies are able to purge the parasite.
So much for contaminated combs and disinfection. Now how about treatment? Oh my gosh, I’m out of space. I’ll pick up again next month with treatments.
citations and notes
[1] Higes, M, et al (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environmental Microbiology, 34(10): 2659-2669.
[2] Chen, Y-W, et al (2012) Nosema ceranae infection intensity highly correlates with temperature. J Invertebr Pathol 111(3):264-7.
[3] Traver, B (2011) Prevalence and infection intensity of Nosema in honey bee (Apis mellifera L.) colonies in Virginia. Journal of Invertebrate Pathology 107: 43 ―49.
[4] https://scientificbeekeeping.com/the-seasonality-of-nosema-ceranae/
[5] https://scientificbeekeeping.com/sick-bees-part-15-an-improved-method-for-nosema-sampling/
https://scientificbeekeeping.com/sick-bees-part-14-an-update-on-the-nosema-cousins/
[6] White, GF (1919) Nosema-Disease. USDA Bulletin No. 780. Available from Google Books.
[7] van der Steen, J, et al (2012) How honey bees of successive age classes are distributed over a one storey, ten frames hive. Journal of Apicultural Research 51(2): 174-178.
[8] Gina Retschnig, et al (2017) Cold ambient temperature promotes Nosema spp. intensity in honey bees (Apis mellifera). Insects 8(1): 20. doi:10.3390/insects8010020
[9] Mulholland, GE, et al (2012) Individual variability of Nosema ceranae infections in Apis mellifera colonies. Insects 2012(3): 1143-1155.
[10] https://scientificbeekeeping.com/sick-bees-part-13-simple-microscopy-of-nosema/
[11] https://scientificbeekeeping.com/sick-bees-part-16-the-quick-squash-method/
[12] Martin-Hernandez, R, et al (2009) Nosema Diagnostic. https://coloss.org/publications/Proceedings_Nosema_Workshop.pdf
[13] Jack, C, et al (2016) Colony level prevalence and intensity of Nosema ceranae in honey bees (Apis mellifera L.). PLoS ONE 11(9): e0163522. doi:10.1371/ journal.pone.0163522
[14] https://scientificbeekeeping.com/sick-bees-part-16-the-quick-squash-method/
[15] Thus some positive spore counts despite not catching any infected bees in the matching sample of 10 bees inspected individually.
[16] Jack, C (2015) Colony level infection of honey bee gut pathogen, Nosema ceranae and role of pollen nutrition in Nosema ceranae infection and bee survival. M.S. Thesis, Oregon State University.
[17] Botías, C, et al (2011) The effect of induced queen replacement on Nosema spp. infection in honey bee (Apis mellifera iberiensis) colonies. Environmental Microbiology 14(4): 845-859.
[18] Simeunovic, P, et al (2014) Nosema ceranae and queen age influence the reproduction and productivity of the honey bee colony. Journal of Apicultural Research 53(5): 545-554.
[19] Furgala, B (1962) The effect of the intensity of Nosema inoculum on queen supersedure in the honey bee, Apis mellifera Linnaeus, J. Insect Pathol. 4, 429 ―432.
[20] White, GF (1919) op. cit.
[21] Smith ML (2012) The honey bee parasite Nosema ceranae: transmissible via food exchange? PLoS ONE 7(8): e43319.
[22] https://scientificbeekeeping.com/nosema-ceranae-kiss-of-death-or-much-ado-about-nothing/
[23] Fries, I., Forsgren, E ( 2009) Nosema ceranae fungerar inte som Nosema apis. Bitidningen 107, Juni, 20-21.
[24] MacInnis, C (2017) Nosema ceranae: A sweet surprise? Investigating the viability and infectivity of the honey bee (Apis mellifera L.) parasite N. ceranae. M.S. Thesis, University of Alberta.
[25] Eischen, F.A., R.H. Graham & R. Rivera (2011) Impact of nutrition, Varroa destructor and Nosema ceranae on colonies in southern Louisiana. Proceedings of the American Bee Research Conference 2011.