The Nosema Problem: Part 4 – Nosemosis
Contents
Effects of nosema upon an individual bee. 1
Effects of nosema upon the colony. 2
Nosema and almond pollination. 4
How concerned should you be about nosema?. 8
.
The Nosema Problem: Part 4
Nosemosis
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in September 2019
Nosema is a common parasite of honey bees, and does not cause any obvious signs of disease. But should the prevalence of infection in the hive get out of hand, then the disease “nosemosis” may become apparent at the colony level, resulting in poor performance, or even depopulation.
Since the invasion of N. ceranae worldwide, we’ve learned a lot more about this parasite and its effects upon individual bees, but are still not completely clear as to its effects upon the colony as a whole.
Effects of nosema upon an individual bee
Although infected bees typically do not exhibit overt signs of disease, they may be negatively affected in a number of ways:
- By damage to the cells lining the bee’s midgut, resulting in impaired digestion.
- By poorer development of the hypopharyngeal glands and fat bodies, and reduced ability to produce jelly as a nurse bee, which may affect protein dynamics for the entire colony.
- By general stress due to infection, and immune suppression by the parasite, which may make the infected bee more susceptible to other stressors or pathogens such as viruses [[1]].
- Infected bees may also exhibit an accelerated transition to foraging behavior, leading to reduced overall longevity. And infected foragers, due to energy issues, may not be able to forage as well.
- The bee’s navigational abilities may also be impaired by infection, resulting in drifting and/or failure to return.
Effects of nosema upon the colony
Nosema, due to its impairment of digestion, and the increase in the protein and energy demands of infected bees, means that those bees may be poorer performers overall, especially with regard to their production of the critical jelly when they are fulfilling their role as nurses. In addition, the infected bees’ reduced overall longevity due to their premature transition to foraging, and then less efficient and curtailed foraging abilities [[2]], result in the inability of a colony to build up its population to its full potential. And if the majority of bees in a colony become infected, the colony may start to spiral downhill (Fig. 1).
Figure 1. One of my colonies, photographed as it was rapidly dwindling in early springtime due to a high prevalence of nosema-infected bees. Note the healthy-looking queen top center, but also a supersedure cell, often found in a heavily nosema-infected hive. Image courtesy Kodua Galieti
Colonies can fight back
My own observations of the impact of N. ceranae upon colony performance agree with those of Dr. White for N. apis ― it’s all about the percentage of bees in the hive infected (the “prevalence” of infection) [[3]]. If fewer than 20% of the workers are infected, I observe little effect. By 40%, I see signs of colony buildup slowing down and decreased honey production [[4]]. At 60% infected, the poorer performance is obvious, and when 80% or more of the bees are infected, the colony can suddenly collapse when it can no longer care for its brood (as in Colony Collapse Disorder). Those percentages are of course approximations, since other colony health factors are often involved.
The critical issue for the colony is then to minimize the transmission of nosema to newly-emerged bees. And this is where is gets complicated ― a colony can rear a lot of new workers when pollen is abundant, and pull ahead of nosema if it has a vigorous queen. But if that pollen is contaminated by nosema spores, or if there is defecation occurring in the hive due to poor flight weather, the rate of infection can increase.
Clearly, the vigor of the queen is critical; but she can also become infected herself (more on this later in this article). Colonies that somehow “sense” that their queen is failing (or that the colony is suffering from a high parasite load or some other issue) will summarily replace their dear mother without hesitation, via the rearing of supersedure cells (Fig. 2). Furgala [[5]] showed that this is the case when the queen becomes infected by nosema.
Figure 2. Should the queen become infected by nosema, the workers may attempt to replace her by rearing supersedure cells. Supersedure is a generic response exhibited by colonies struggling with any number of issues. With a new queen, and better flight weather, the colony may be able to break the infection cycle, and purge nosema from the hive.
Practical application: Despite the fact that nosema proliferates during times of pollen abundance (more on this later), a colony with a large enough population and a vigorous queen [[6]] with plenty of room for egg laying, may be able to rear new workers fast enough to stay ahead of the accelerated attrition of infected workers ― provided that the fecal-oral route of spore transmission is reduced due to there being adequate opportunity for defecation flights.
Nosema and almond pollination
Early this winter some beekeepers paid me to test a substance to control nosema. I began the trial at the end of January (after the colonies had been rearing brood for nearly three weeks), by taking samples of bees from an outer comb from 25 hives ― I intentionally chose medium-strength hives for the trial, since I assumed that they would be more likely to exhibit some degree of nosema infection. To my great surprise, we only spotted a few (sometimes questionable) spores in only a few of the bee samples — nosema appeared to have disappeared during our brief winter brood break in the California foothills.
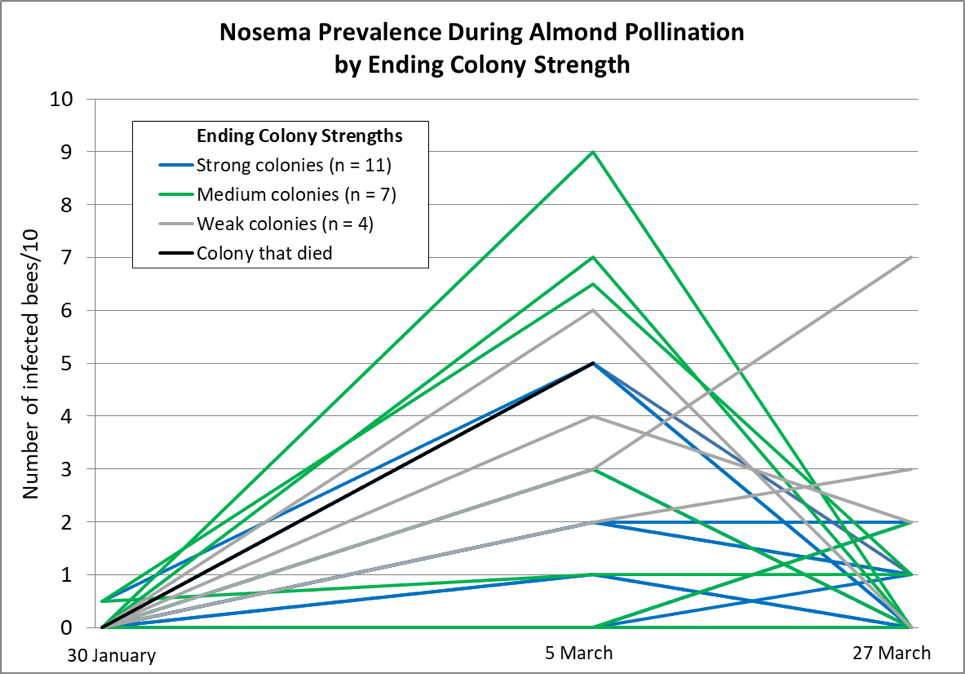
We then gave all the hives pollen sub and moved them to the almonds, where they then suffered through the rainiest bloom in memory, but were able to fly for a short period of time most days. This combination of pollen income coupled with cool weather and greatly reduced flight hours was not favorable for colony buildup, but certainly conducive to nosema buildup. So it wasn’t unexpected that when we tested the same hives 34 days later in early March, the median nosema prevalence had risen considerably (ranging from 0-9 bees/10) (Table 1 & Fig. 3).
We initially scored colonies as 0.5 if we saw the rare spore in a composite sample of 10 bees; if we saw more than a single spore, we then individually crushed 10 new bees from the same sample. The trial was to determine whether the test substance reduced nosema prevalence (unfortunately, there was no apparent effect from treatment); measuring colony strength was not part of the protocol. But at final grading, there was so much difference in colony performance, that I noted whether each colony at that time was of strong, medium, or weak strength by a quick eyeball grading.
Note that even some of the strongest colonies coming out of almonds had reached a prevalence of half the bees being infected by end of bloom, yet both the strong and medium groups largely purged their infections by the end of March. I’ve graphed the results below.
Figure 3. Nosema prevalence rose during the almond bloom, and then tended to decline toward the end of bloom (the bloom extended well into March). The chart does not make clear that a number of colony plots overlap. Note how the weak colonies (gray lines) and dead colony (black line) tended not to get nosema under control.
We took around 1000 hives to almonds. Since I had intentionally chosen weaker hives for the nosema trial, the hives above were fairly representative of the poorer hives that we took. The cool, wet weather definitely set our colonies back during almond bloom, and it continued through March, with the bees confined to the hive for days or weeks at a time — meaning that conditions were favorable for nosema to go wild in the hives. And although our hives returned from almonds weaker than normal, only a handful had died (although a fair number had barely grown). Yet when the weather broke in the second week of April, our main problem was that nearly all of our colonies had built up to swarming strength and were ready to hit the trees, indicating that nosema just didn’t seem to be that big an issue in the long run.
Practical application: Go figure — under the cold, rainy conditions this late winter and spring, with adequate pollen and little opportunity for defecation flights — a situation certainly conducive to nosema reproduction — our colonies did not suffer notable mortality, nor even a major setback in their buildup once good weather returned.
I’ve also in recent years been checking my cell builder colonies when we have trouble rearing queen cells ― again, I do not see nosema as being prevalent. And this brings us to the queens themselves.
Nosema and the queen
What’s difficult to make sense of is the history of nosema. Back in 1947, Farrar [[7]] presented compelling data that N. apis was infecting most package bees, that the queens were easily infected, and that infected queens would soon fail:
The first queens examined for Nosema infection were removed from their colonies in June 1941. They had laid normally for about 2 months, but suddenly stopped laying, became sluggish, and most of their last eggs shriveled and failed to hatch. All were found to be heavily infected with Nosema. Previously, many queens had been observed to manifest similar symptoms, but the superseded queens either were not found or they were not examined for Nosema.
Something that is seldom discussed is that the problem with a queen becoming infected may not only be a decrease in her ability to produce eggs, but that she’s the only bee that routinely defecates within the hive.
Practical application: Since the queen’s feces are consumed by the attendants around her, an infected queen can become a “Typhoid Mary” ― a source of inoculum to her daughters, for as long as that queen continues to survive. This has been studied to some extent with N. apis, but more research regarding the extent that this occurs with N. ceranae would be informative.
One would think that nosema would have continued to be widespread through the U.S. But compare Farrar’s observations to the USDA Diagnostic Lab data that I showed in Part 2 of this series *(July 2019 ABJ), which indicated that during the period from 1984 clear until 2002, nosema was rarely detected in samples of sick bees sent to the lab for analysis. Had our bees developed some degree of resistance to nosema?
It appears to me that if our bee stocks had indeed developed some resistance to N. apis, that such resistance did not appear to apply to N. ceranae, as the invasive wave of this version clobbered our industry from 2004 through around 2009 (during the CCD epizootic). A number of studies have demonstrated that N. ceranae is indeed able to infect queens [[8], [9], [10]]. So why don’t we see the queens rapidly dying in colonies infected with N. ceranae, as did Farrar with N. apis? I can’t cite definitive data, but personal observations and some published studies [[11]] suggest that while N. ceranae may infect queens, that it may not do so as readily as did its cousin (again, we need more research along this line).
Practical application: My sons and I rear thousands of queens each season, and N. ceranae can generally be found in our colonies in spring. Yet we don’t notice any great degree of queen failure, and our nucs typically grow rapidly (although we often see successful early supersedure in some groups). This spring I couldn’t make any connection between laggard nucs and nosema prevalence. Even in their second season, our queens tend to be productive and healthy, until they apparently start running out of stored sperm late in their second summer, as would be expected.
When we have trouble with rearing queen cells, I check my cell builders for nosema ― I rarely see more than minor infection prevalence in the house bees. And when I check what appear to be poorly-performing queens under the ‘scope, I don’t find any infected by nosema. I occasionally hear reports of commercial queens failing at a young age, but have yet to see evidence that N. ceranae is causing the sort of queen and package failure that was previously associated with N. apis.
How concerned should you be about nosema?
In his review on N. apis in 1993 [[12]], Dr. Ingemar Fries concluded that:
- apis infections are frequently present in most apiaries without causing significant damage. Except for the infective agent, contributing factors in the environment decide if the infection develops into an epizootic disease. Thus, nosema disease can be regarded as a factorial disease.
It appears to be the same for N. ceranae, although ceranae may pop up in warm weather. The factors that Fries listed were:
- Colony crowding and stress.
- Disturbance of the hive during winter, or the moving of hives early in the season.
- Lack of flight opportunities leading to defecation within the hive (Fig. 4).
And similarly to others, he noted that the availability of good pollen sources, or supplementary feeding of protein, “reduces the infection level in infected colonies, probably due to a larger number of bees being produced.”
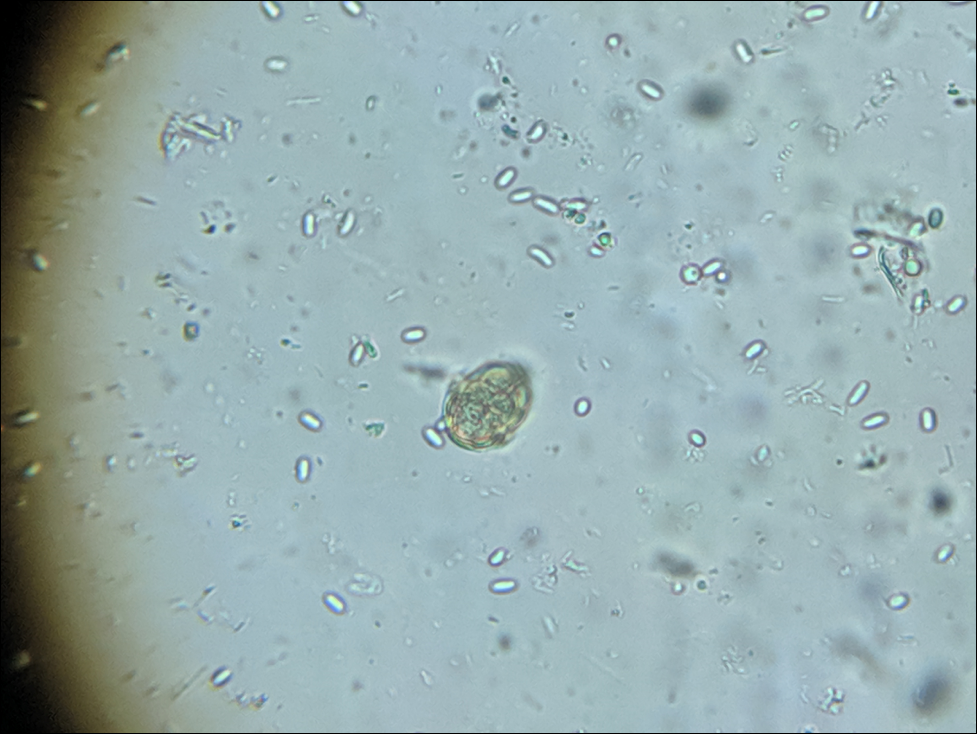
Figure 4. A friend sent me a sample of dead bees swept from the floor of his large winter storage shed. This diluted composite sample was loaded with spores (enlarged from 400x, with a pollen grain for reference). Storing hives indoors at a controlled temperature of around 41°F (5°C) allows infected workers to leave the colonies for defecation flights, although they don’t make it back to the hive. This would prevent them from defecating spores within the hive, and could be one of the major benefits of controlled-temperature indoor wintering.
Practical application: I’ve reviewed studies from all over the world [[13]], and my impression is that nosema is an opportunistic parasite that thrives when colonies are bringing in lots of pollen, and that tends to disappear when they are not, and is not normally the cause of colony mortality. Perhaps surprisingly, the above appears to apply even in cold-winter climates, as evidenced in both Germany and Canada:
The results of our study failed to reveal a relation between N. ceranae infection of colonies and colony mortality, even in seasons with unusually high colony loss rates. Likewise, monitoring of the fate of individual N. ceranae-infected colonies over several years did not show a mandatory link between this infection and failure of the colony [[14]].
No differences in Nosema infection levels were found between colonies that died and those that survived [the winter in Ontario, Canada] [[15]].
The above findings do not let nosema completely off the hook, as it may get out of hand in colonies stressed by lack of flight weather, cold (especially if the colony is weak), poor nutrition, brood disease, or varroa. Other contributing stressors can be a co-infection by viruses or other pathogens [[16]], treatment with an antibiotic [[17]], or perhaps certain pesticides. The major factor appears to be whether for one reason or another, much defecation is taking place in the hive.
The take-home message
At least in California, once the initial invasive wave of Nosema ceranae passed through, nosema no longer appears to be something for me to particularly worry about. When I tracked its prevalence and spore counts in 36 hives over the course of the year, I found only a slight negative correlation with colony performance [[18]]. But as in my experiment in almonds detailed above, I do see that weak colonies can indeed get hammered by nosema during cold or rainy weather. Researchers around the world have now confirmed that infection by N. ceranae, similar to that of N. apis, typically peaks during cool weather [[19]] or early spring, and tends to disappear in summer. Nor is it necessarily correlated with winter loss — even in northern climes. However, infection may reduce colony productivity.
Keep this in mind when you think of nosema: Unlike American and European Foulbroods, which need to kill their host in order to gain transmission of their offspring to new hosts, there is no benefit to nosema to kill or harm its host bee, nor the colony. N. apis appeared to be a relatively benign parasite (unless it infected the queen), rarely killing infected colonies unless they were confined in early spring for long periods by cold or inclement weather (Fig. 5). N. ceranae appears to me to be much the same, other than perhaps being more prevalent during summer (not surprising, since it appears to be adapted to tropical environments).
Figure 5. A rapidly-growing colony with plenty of pollen can shrug off having a small percentage of its workers infected by nosema. However, if the beekeeper were to sample foragers at the entrance, they might freak out at the spore count. I’ll elaborate in my next article.
Nosema, in and of itself, may not normally cause serious disease, but rather it is about external nutritional and weather factors that can tip the scale toward it going epizootic in the hive. For example, in the eastern U.S., a bout of early warm weather along with tree pollens can cause the diutinus “winter bees” to start consuming pollen and shift to nurse physiology. This would tend to kickstart nosema reproduction in those unfortunate geriatric bees. But if that burst of broodrearing was then curtailed by the return of cold weather (which might also prevent defecation flights), then the colony would be unable to continue to replace the infected bees with fresh workers, and nosema could then be problematic.
Practical application: Since nosemosis exhibits no obvious signs of the disease, it behooves the beekeeper to better understand this parasite, its seasonality, and the factors that contribute to it going epizootic in a hive. I’ve seen far too many beekeepers dosing their hives with unnecessary (and possibly harmful) treatments, without having ever spent a few minutes to confirm that nosema was actually prevalent in their hives.
Coming
In my next articles I’ll cover monitoring for nosema, dealing with deadout equipment, treatments, and the causes of dysentery.
citations and notes
[1] Robinson, C & J Pfeiffer (2014). Viruses and the microbiota. Annual Review of Virology 1: 55―69.
[2] Dussaubat , C, et al (2013) Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. Journal of Invertebrate Pathology 113: 42―51.
[3] White, GF (1919) Nosema disease. U.S. Dept Agric Bulletin 780, 59 pp. Available in Google Books.
[4] https://scientificbeekeeping.com/nosema-ceranae-and-honey-production-in-healthy-colonies/
[5] Furgala, B (1962) The effect of the intensity of nosema inoculum on queen supersedure in the honey bee, Apis mellifera Linnaeus. Journal of Insect Pathology 4(4): 429-432.
[6] Botías, C, et al (2011) The effect of induced queen replacement on Nosema spp. infection in honey bee (Apis mellifera iberiensis) colonies. Environmental Microbiology 14(4):845-5.
[7] Farrar, C (1947) Nosema losses in package bees as related to queen supersedure and honey yields. J Economic Entomol 40: 333―338.
[8] Higes M, et al (2009) Horizontal transmission of Nosema ceranae (Microsporidia) from worker honeybees to queens (Apis mellifera). Environ Microbiol Rep. 1(6): 495―498.
[9] Simeunovic, P, et al (2014) Nosema ceranae and queen age influence the reproduction and productivity of the honey bee colony,.Journal of Apicultural Research, 53(5): 545-554.
[10] Alaux, C, et al (2011) Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). Journal of Invertebrate Pathology 106(3): DOI : 10.1016/j.jip.2010.12.005
[11] Botías, C, et al (2011) The effect of induced queen replacement on Nosema spp. infection in honey bee (Apis mellifera iberiensis) colonies. Environmental Microbiology 14(4): 845-59.
[12] Fries, I (1993) Nosema apis — A parasite in the honey bee colony. Bee World 74(1): 5-19.
[13] Genersch, E, at al (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie DOI: 10.1051/apido/2010014
Traver, B & R Fell (2011) Prevalence and infection intensity of Nosema in honey bee (Apis mellifera L.) colonies in Virginia. Journal of Invertebrate Pathology 107(1): 43-49.
Traver, B & R Fell (2012) Comparison of within hive sampling and seasonal activity of Nosema ceranae in honey bee colonies. Journal of invertebrate pathology 109(2): 187-193
Invernizzi, C, et al (2009) Presencia de Nosema ceranae en abejas melíferas (Apis mellifera) en Uruguay. J. Invertebr. Pathol., 101: 150-153.
Williams, G, et al (2010) Effects at Nearctic north-temperate latitudes of indoor versus outdoor overwintering on the microsporidium Nosema ceranae and western honey bees (Apis mellifera). Journal of Invertebrate Pathology 104: 4―7.
Williams, G (2013) Nosema ceranae in western honey bees (Apis mellifera): biology and management. PhD Thesis, Dalhousie University.
Gina Retschnig, et al (2015) Effects, but no interactions, of ubiquitous pesticide and parasite stressors on honey bee (Apis mellifera) lifespan and behaviour in a colony environment. Environmental Microbiology doi:10.1111/1462-2920.12825
Compare the above to the publications of Mariano Higes et al., 2007-2009.
[14] Gisder, S, et al (2010) Five-year cohort study of Nosema spp. in Germany: does climate shape virulence and assertiveness of Nosema ceranae? Applied and Environmental Microbiology, 76: 3032―3038. http://dx.doi.org/10.1128/AEM.03097-09
[15] Guzmán-Novoa, E, et al (2010) Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 41: 443―450.
[16] Cornman, RS, et al. (2012) Pathogen webs in collapsing honey bee colonies. PLoS ONE 7(8): e43562. doi:10.1371/ journal.pone.0043562
[17] Li, JH, et al (2017) New evidence showing that the destruction of gut bacteria by antibiotic treatment could increase the honey bee’s vulnerability to Nosema infection. PLoS ONE 12(11): e0187505. https://doi.org/10.1371/journal.pone.0187505
[18] https://scientificbeekeeping.com/the-seasonality-of-nosema-ceranae/
[19] Chen, Y, et al (2012) Nosema ceranae infection intensity highly correlates with temperature. J. Invertebr. Pathol. 111: 264―267.