A Test of Using CO2 for Bee-Friendly Mite Monitoring
A Test of Using CO2 for Bee-Friendly Mite Monitoring
First Published in ABJ April 2017
Randy Oliver
ScientificBeekeeping.com
Beekeepers who monitor the varroa level in their hives tend to be more successful at keeping their colonies alive and healthy. But no one likes having to sacrifice bees to take mite counts. So when I heard of using CO2 to temporarily anesthetize the bees in a mite shaker I was intrigued.
Introduction
For most beekeepers (other than the minority running resistant stock), our worst enemy is the varroa mite, which if not controlled, will build up to a level that initiates a virus epidemic, thus causing the collapse of the colony. The best way to assess the status of the mite infestation of your hives over the course of the season is by monitoring. Such monitoring requires some method of accurately determining the degree of mite infestation. Most researchers express this as mites per 100 (adult) bees, or percent infestation (e.g., 3 mites in a 300-bee sample would indicate a 1% infestation rate) [[1]].
In general, colonies on good forage and engaged in active broodrearing can easily tolerate a mite count of 2 mites/100 adult bees taken from near the broodnest [[2]]. Under favorable conditions, a healthy colony may be able to tolerate up to 5 mites/hundred, due to the rapid overturn of the adult bee population, but the performance of that colony will not be up to its full potential. The main problem with varroa typically occurs in late summer and fall, when the mite population appears to explode as the roughly 50% of mites that were hidden in the brood are forced onto the adult bees. Once the infestation rate reaches a ballpark figure of around 20 mites/100, most colonies will exhibit the telltale signs of PMS (parasitic mite syndrome), and soon collapse.
Practical application: the point is for the beekeeper to track the trend of mite increase, realizing that it will generally increase at a predictable and exponential rate over the course of the season (roughly doubling each month–I will be writing about this rate in the near future). One should aim to keep the infestation rate at less than 1 mite/100 early in the season, perhaps allowing it to climb to 2-3 mites/100 in late summer. Colonies entering winter with an infestation rate of over 5 mites/100 rarely survive ‘til spring—better to get the mite count way down by September.
I’ve written previously about monitoring methods [[3]]. The Gold Standard is the alcohol wash, with which one can obtain 100% recovery of the mites in less than a minute of gentle agitation [[4]]. The unfortunate downside of the alcohol wash is that you need to sacrifice 300 bees (a trivial number, compared to allowing all 30,000 bees in the hive to die in the fall from varroa). That said, I take no joy in killing those bees, and always fear the risk of inadvertently killing a queen.
An alternative is the powdered sugar shake, which, if done properly, can result in over 70% recovery of the mites, and although involving rather brutal abuse of the bees (especially in hot weather), may not injure them too badly. But be aware that the poor rate of recovery attained by a less than perfectly-performed sugar shake can result in missing a serious infestation (I see this happen frequently), plus the amount of hard shaking required is tough on the arm (tip: use a plastic jar).
Stickyboard counts of natural mite fall, although excellent for tracking the efficacy of a treatment, can be very misleading. As I was typing these words, I ran out to check the stickyboard that I had placed under the hive used for the experiment in this article. I know that that colony is suffering from a sky-high infestation rate of 20 mites/100, is exhibiting signs of PMS, and would be expected to soon collapse completely, yet when I put on my reading glasses, I was able to tediously discover only 42 mites in the hive trash on the board—a 24-hour count lower than the commonly recommended treatment threshold of 50-60 mites in autumn [[5]].
I’d recently seen reports promoting the use of CO2 in mite shakers, rather than alcohol or powdered sugar. What a great idea, I thought—no mess, no killing of bees, I need to try it! But my testing unfortunately got shoved to the back shelf until David Waldman of the New Jersey Beekeepers Association funded me to test a CO2 shaker manufactured in Denmark—called the Varroa-Tester.
 This attractively designed mite shaker was very appealing, so I checked out the product description at Swienty’s website [[6]]. There was a video on how to use it [[7]]—it looked like nothing could be simpler. So going into my testing, I had high hopes for the method.
This attractively designed mite shaker was very appealing, so I checked out the product description at Swienty’s website [[6]]. There was a video on how to use it [[7]]—it looked like nothing could be simpler. So going into my testing, I had high hopes for the method.
Practical application: I’d love to find a quick, non-messy, accurate method for monitoring mites that doesn’t involve killing bees. CO2 certainly appeared to hold great promise.
 The Varroa-Tester has a cute graphic on the lid explaining how to use it.
The Varroa-Tester has a cute graphic on the lid explaining how to use it.
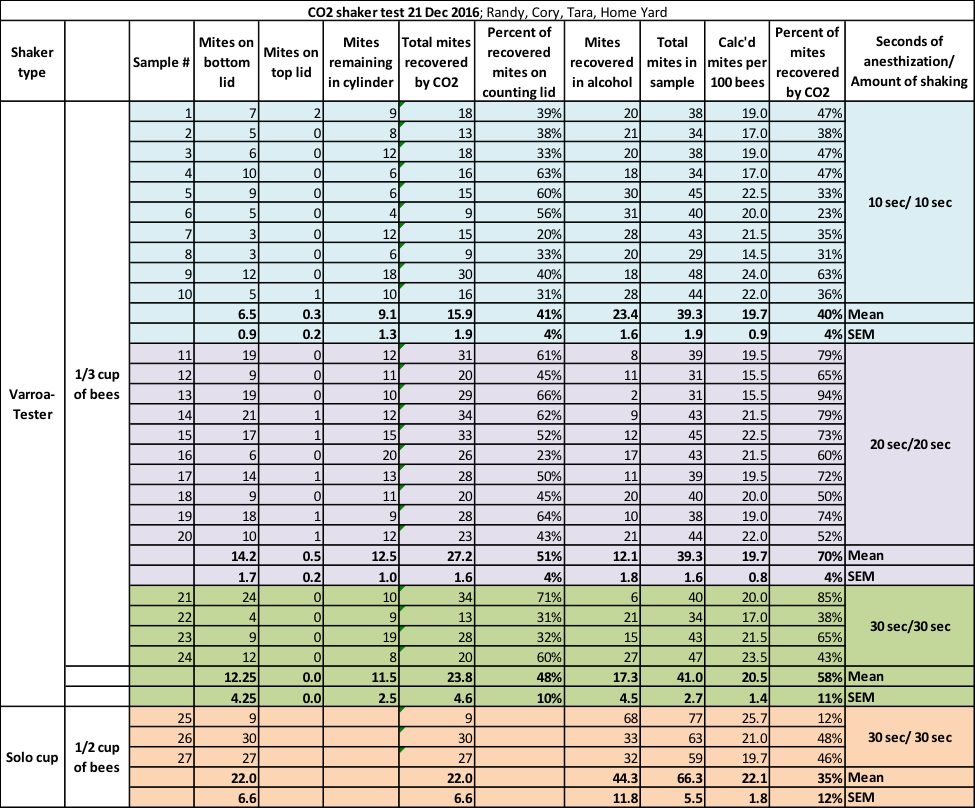
For uniformity, we (I was assisted by Cory Dodgson and Tara McKinnon) took all the bee samples from a single strong colony with a very high mite infestation (and showing signs of PMS) on December 21st, shaking the bees from broodnest frames (the colony was in the process of shutting down broodrearing). Note that in the photo above, I am shaking the comb of beebread adjacent to brood.
Since the varroa-preferred nurse bees need a steady diet of beebread, such a comb is suitable for taking bee samples. I intentionally chose a colony with a high mite count in order to obtain better statistical data. We performed the tests in late afternoon. Ambient temperature was around 60°F, and the bees were warm and moving freely by the time they made it into the cup.
We scooped 1/3 cup of bees (roughly 200) from the tub for each sample, since we found that it was difficult to use the red lines on the shaker bottle as an accurate measure (since the bees simply don’t want to sit still). We normally sample ½ cup of bees (roughly 300), but for this test we followed the Varroa-Tester’s instruction to use 200 bees in their shaker.
We dumped the bee sample into the shaker and snapped on the lid. By shaking the bees first into a tub, we allowed older bees to fly off, thus ensuring that we sampled only younger bees with the most mites.
We then injected low-pressure CO2 until the bees became anesthetized and dropped to the bottom of the container—this only takes a few seconds. At that point we’d then wait for the prescribed number of seconds (for the mites to be fully exposed to the CO2) before we began shaking.
As with many experiments in which I’m testing something for the first time, ideas to improve the experiment arise only after we start. This experiment was no exception, and evolved after we’d shaken the first couple of samples. We questioned whether the recommended 10 seconds of shaking was enough agitation to allow all the mites to drop through the perforated sieve plate. I decided to divide the experiment into three tiers of exposure to CO2, as well as the number of seconds of shaking, in order to determine whether those variables affected the method’s success at mite recovery:
Tier 1 (10 samples): As per the instructions on the shaker—a 10 sec wait after the bees became immobilized by the gas, followed by 10 seconds of shaking (we shook with at least the degree of vigor shown on the video).
Tier 2 (10 samples): 20 sec CO2 exposure after immobilization, 20 sec shaking.
Tier 3 (4 samples, since we had taken only 24 alcohol-filled sample jars to the test yard): 30 sec CO2 exposure after immobilization, followed by 30 seconds of shaking.
After waiting for the prescribed number of seconds (for the CO2 to take full effect upon the mites), we then shook the bees for the appropriate number of seconds. Since the point of using CO2 was to not harm the bees, we shook vigorously enough to dislodge the mites, but not hard enough to injure the bees.
Practical application: One problem with alcohol wash shakers made from two joined jars is that if one shakes up and down, with each shake the slosh of alcohol carries washed-off mites back up above the bees, where about 15% of them then get filtered out by the bees’ bodies as the alcohol runs back down (this is why we now use a swirl-type alcohol wash shaker). With CO2 this doesn’t occur—the mites don’t have enough body mass to be lifted off the bottom of the container on the downstroke. Nevertheless, we also tried side-to-side shaking, but observed no noticeable difference in the number of mites dropped.
At this point we were really excited—the CO2 method was really quick, easy, not messy, and after shaking off the mites, we could dump the bees back onto the landing board of the hive, after which they soon recovered and walked back in. What could be easier or more bee friendly?
Above I’m pointing a pencil towards mites clinging to the side of the shaker.
Unfortunately, our exuberance was tempered by a few annoying details. In the inventor’s video, he counts only the mites on the bottom lid. But we quickly found that only a portion of the mites drop there. Roughly half of the dislodged mites somehow cling to the sides of the shaker, the top lid, or to the perforated sieve plate.
Many mites clung to the top or bottom of the aluminum sieve plate. This is a serious design flaw–fewer mites did this when we tested using a screen instead of the perforated plate.
What was really annoying was the number of mites that wedged under the notched plastic ring that attached the sieve plate to the cylinder. These mites were really difficult to dislodge between samples—they could not be shaken or banged out.
Practical application: if you counted only the mites on the bottom lid (as demonstrated in the video), you’d underestimate the number of dislodged mites by half (and this is even before allowing for the success of the method at dislodging mites in the first place). It took considerable time and effort to brush those clinging mites out of the shaker between samples.
After the CO2 shake, we removed the top and bottom covers, then gently dumped the still-immobile bees into alcohol for later recovery of any mites that had not yet been dislodged from their bodies. We then separately counted the number of mites on the lower lid, the upper lid, or remaining in the cylinder.
It was such a pain to dislodge mites from the cylinder and sieve plate, that I brought up compressed air to blow out the clinging mites after we’d counted them. This worked well, but would be unavailable in most outyards.
After we finished collecting data for 24 samples, and being disappointed by the amount of work required to remove the mites from the cylinder, and decided, for comparison with the Varroa-Tester, to shake a few samples using our standard Solo® alcohol wash cups [[8]], but using CO2 instead of alcohol. These worked great! Far fewer mites left in the cups, better drop through the screen, easier to shake (no splashing, and less weight than with alcohol), easier to count, and again, unharmed bees. I was really excited, and started figuring out how we were going to switch over to using CO2 instead of alcohol.
There was only one remaining thing to do—to wash the 24 bee samples in alcohol to confirm that CO2 shaking indeed resulted in an acceptable level of mite recovery.
My field setup for mite washing. I obtain 100% recovery of mites with this method. We washed each numbered sample to recover any mites remaining on the bees after the CO2 shake. To our great disappointment, we found that CO2 and moderately vigorous shaking doesn’t do a very good job at causing mites to release from the bees:
The recommended 10-sec CO2 wait and 10-second shake recovered, on average, only a disappointing 40% of the mites. The 20-sec timing appeared to be more efficient, with 70% average recovery, but this figure is questionable, since the 30-sec shake only resulted in only 58% recovery.
Practical application: I repeat this again and again—buyer beware! The folk who write ad copy for selling a product may not have supportive data for their claims.
Discussion
If you followed the directions found on the Varroa-Tester lid, and only counted the mites on the catch lid (as demonstrated in the video), you’d only be counting about 16% of the total mites in the bee sample [[9]]—thus seriously underestimating the colony’s actual mite load by a factor of six (meaning that if you saw even a single mite on the lid, that your hive was already in trouble).
Practical applications:
- The results of our testing strongly suggest that mite counts obtained using the Varroa-Tester per instructions and as demonstrated in the video would greatly underestimate the actual degree of mite infestation of the bees.
- However, by increasing the wait time to 20 seconds, and then shaking for 20 seconds, and then counting every mite on every surface of a sticky shaker (see below), you might recover about 70% of the mites. This would underestimate the actual infestation rate by only 30%, which could be allowed for. For example, if your treatment threshold was 2 mites/100, and you got 70% recovery from a 200-bee sample, then you’d treat if you recovered 2 or more mites in total (on the lids, sides, and wedged in the sieve plate).
- The removal of mites from the Varroa-Tester between samples is problematic. Perhaps one could follow the CO2 shake (after dumping the bees) with a soapy water or alcohol wash of the shaker to recover all the dislodged mites for counting.
- Note the trend of higher mite recovery from samples 1–24—the number of mites remaining in the cylinder tended to increase over time. We started with a brand new unit, and never washed it during the testing—it got a little sticky inside. Perhaps this stickiness helped to recover mites from the bees–their clinging to the sides of the container is similar to that which takes place in an ether roll (in which the mites stick to the disgorged nectar on the sides of the jar). Unfortunately, if one were to wash the mites from the shaker each time, that would remove the stickiness.
- We greatly preferred the home-made Solo® cup mite shakers to the Varroa-Tester —they were much easier to hold and use, there were fewer mites on the sides (until they too got sticky), and after dumping out the bees, any stray mites could be easily washed into the bottom cup for counting with a little soapy water or diluted alcohol.
Although I was disappointed with the Varroa-Tester, have I given up on CO2 for mite monitoring? Hardly! With experimentation by beekeepers, the 70% recovery rate might well be improved on. And although a 70% recovery rate would not be acceptable for scientific data, it’s enough for general monitoring purposes.
Another expert concurs
After performing my testing, I found that Dr. Ralph Büchler of Germany had written about the Varroa-Tester in 2015 [[10]]. He also found that it was not accurate enough for scientific use, but felt that the method, if used every few weeks, had practical value for monitoring whether colonies needed treatment (he also suggested that tinkerers build their own cheaper version).
Practical application: experimenters, I’m putting out the challenge: let’s figure out how to make CO2 work for mite monitoring. I’ve demonstrated how to do the testing for confirmation of efficacy. Let’s work together to come up with a better method. Send me your improvements, and I’ll update this article at ScientificBeekeeping.com.
A side note on sampling consistency
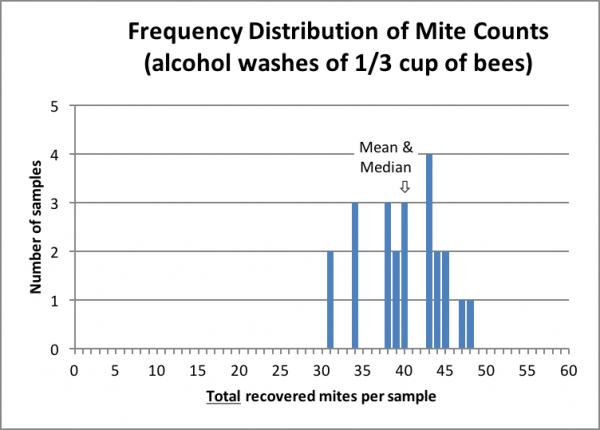
As I began working up the data from this experiment, I belatedly realized that we had unintentionally also collected a nice data set for testing the consistency of mite distribution on bees in the hive. All our bee samples were taken from one or more of three frames at the edge of the broodnest from the same hive, only two of which contained any brood (we shook bees several times over the course of two hours of testing, replacing the frames into the hive, allowing fresh bees to repopulate them, and then shaking the same frames again). Thus, we obtained final alcohol wash mite counts, from the same hive, for 24 individual samples of roughly 1/3 cup scoops of bees (we hadn’t made any effort to measure exactly).
Since the number of bees in each sample was relatively small (~200 bees), the data set above lends itself well to detecting the degree of variation in mite infestation among the workers. Since this was late season, nearing the end of broodrearing, such information would be useful to those monitoring their mite levels prior to entering winter.
There was high uniformity in the distribution of the 24 mite counts (despite the fact that we hadn’t made any effort to level the bees in the measuring cup)—all were within 25% of the mean/median value (only 2 of 24 samples underestimated by 25%). There would likely have been less variability had we taken 300-bee samples or measured more accurately.
Should the same degree of variability hold for lower mite infestation rates (say at around the BIP action threshold for treatment of 3 mites/100), then had the median value of the 24 samples been 3 mites/100, then none of the 24 samples would have been below 2/100 or above 4/100.
Practical application: this data set again confirms that a single bee sample from the edge of the broodnest is a reliable way to monitor the degree of mite infestation in a hive.
Acknowledgements
Thanks to J. David Waldman, member of Northwest New Jersey Beekeepers Association, for funding this research.
Notes and Citations
[1] If you take a ½-cup sample of bees (roughly 300 bees), divide the mite count by three in order to obtain the infestation rate per 100 bees (percent infestation). This number reflects only the infestation rate of the adult bees, but serves as a proxy for the overall impact of mites upon the colony, and correlates with the degree of virus vectoring by the mites.
[2] It is not necessary to take the bee sample from a brood frame. The young bees favored by mites are found throughout the brood chambers, and if one shakes a frame of bees into a tub, the older bees will immediately fly off, leaving only the younger mite-preferred bees behind for you to sample.
[3] https://scientificbeekeeping.com/varroa-management/mite-monitoring-methods/
[4] Swirling or side-to-side agitation, not up and down shaking.
[5] http://www.brushymountainbeefarm.com/Resources/VarroaMites.asp
[6] http://www.swienty.com/shop/vare.asp?side=0&vareid=115879
[7] https://youtu.be/c1HCTB6mVEE Note: I notified Swienty that this video was misleading; they may have taken it down by the time you read this.
The same video is on the inventor’s website at (Broken Link!) http://ellumbiavl.dk/da-dk/varroa-tester/
[8] https://scientificbeekeeping.com/an-improved-but-not-yet-perfect-varroa-mite-washer/
[9] 40% x 41% = 16%
[10] Büchler, R (2015) „Varroa-Tester“ getestet. ADIZ • die biene • Imkerfreund 09.201 http://media.repro-mayr.de/86/641286.pdf