The Varroa Problem: Part 2 – The Coevolution of the Bee, Varroa and DWV
The Varroa Problem: Part 2
The Coevolution of the Bee, Varroa, and DWV
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in Dec 2016
In order to solve The Varroa Problem, we need to understand the biology of how it was created, and what perpetuates it.
A Time To Work Together
I’m a strong proponent of using regionally-adapted bee stock, bred for resistance to the varroa/virus complex. My dearest dream is to keep bees that manage mites on their own, but I’ll be the first to admit that I’m still forced to use treatments to keep my hives from crashing [1].
Although my goal is “treatment free” beekeeping, I’m concerned about beekeepers who use “treatment free” as being an excuse for negligence of animals under their care, or who are doing so without a clear understanding of its potential negative impact upon the natural evolutionary process, or who are unaware of the serious repercussions that their collapsing colonies have upon neighboring beekeepers.
Since I make my living from a commercial operation, I completely understand the problems faced by professional beekeepers, and feel that they have been undeservedly criticized. Although I use only organically-approved treatments myself, that is only due to personal preference (and as a sales benefit to differentiate my hive products from the competition’s)—I have no criticism of the professionals who use more effective synthetic miticides, so long as they don’t taint the good image of honey.
I’m giving this introduction in order to make it clear that in this series I have no axe to grind, criticisms to make, nor agenda to promote, other than to help my readers to better understand varroa and its management, and to offer scientifically-based suggestions as to how we can all work together to resolve The Varroa Problem.
The Heart Of The Problem
Having a few mites in the hive isn’t a problem for the colony. Varroa only becomes a problem if the bees don’t keep the mites in check–allowing them to reproduce to the extent that they reach a harmful level. That level used to be surprisingly high (50,000 or more mites), and a single mite treatment in fall would allow the colony to recover. Unfortunately, as viruses—notably Deformed Wing Virus (DWV)—evolved to take advantage of varroa as a vector, the level at which varroa harms the colony has now gotten much lower (~1000 mites going into winter). So when we discuss The Varroa Problem, we are really dealing with two symbiotic parasites—varroa and DWV.
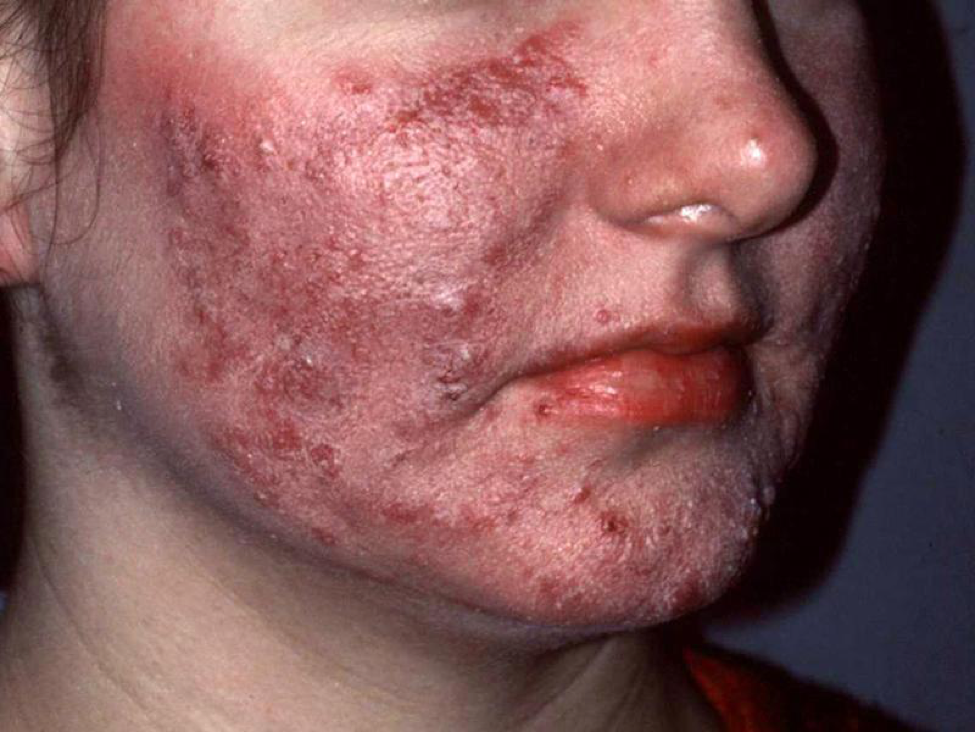
In nature, host-parasite relationships either reach some sort of equilibria, or the host (or parasite) goes extinct. Some parasites reach a steady equilibrium state with a host. For example, every adult human (yes, you) is infested with mites—tiny follicle mites that are a normal component of our skin microbiome. The population of these mites normally reaches an equilibrium that does not cause discomfort to us, but if our immune system does not keep them in check, they can cause visible disease (Fig. 1).
On the other hand, some host-parasite relationships oscillate over the years—in my local forests, when the Gray Squirrel population builds up to high density, we start to see scraggly sick squirrels, infested with mites and nematodes. The density-dependent transmission of these parasites results in much of the squirrel population dying off. At that point, the parasites also diminish, and the few surviving squirrels steadily expand their population before the parasites catch up again. This repeating oscillation of the squirrel population is the norm.
Note: keep the above density dependence in mind, as it turns out to be a critical factor in the evolution of mite resistance in the honey bee.
On its original host, the Eastern honey bee (Apis cerana), varroa exists in a stable host-parasite relationship with the bees—it’s always present, but held in check by the host [3]. When humans first brought the Western honey bee (Apis mellifera) into contact with varroa, the mite got transmitted into its hives, but was not adapted to this novel host, and for many years did not cause problems. It was apparently only after a chance mutation in a single mite that the pandemic invasion of the Korean haplotype of Varroa destructor (apparently all descendants of that original mutant) began [4]. Our unfortunate bees are now living with the consequences, and we are party to watching the coevolutionary establishment of a novel host-parasite equilibrium.
The Mutation That Started The Varroa Problem
So what was that mutation that resulted in varroa becoming a problem in A. mellifera? Surprisingly, I seldom hear beekeepers or researchers discussing that critical mutation. The reader may wish to pause for a moment to reflect upon this…
Did you figure it out? OK, let me introduce to a couple of terms. A parasite can have various hosts over the course of its development. For example, deer tick larvae feed on lizards, mice, and birds; tick nymphs feed on deer mice, dogs, or people; adult ticks feed only on large mammals. But it is only on the large mammals that ticks reproduce. Thus, the lizards, mice, and birds are called intermediate hosts, and deer are called the definitive host—the host upon which the parasite depends upon for reproduction.
In A. cerana colonies, the adult bees serve as intermediate hosts, and the definitive host is the drone pupae—varroa does not reproduce on worker pupae to any extent. The same occurred when varroa was first introduced to A. mellifera—the female mites were attracted only to drone brood (Fig. 2), and would not normally be triggered to ovulate if they entered a worker cell [5]. Thus, the mite was not initially a problem in A. mellifera, since the amount of drone brood was the limiting factor for their reproduction [20].
“The Mutation” was apparently a slight shift in one mite’s triggering mechanism for ovulation [6], allowing that female to respond to the cue of worker brood, and thus successfully reproduce there. This then gave the mite two definitive hosts—both the drone brood and the worker brood.
Practical application: A. cerana limits mite reproduction by restricting the amount of drone brood in the hive (as well as using a number of other mechanisms which I will later describe). A. mellifera is unable to limit the amount of worker brood (if the colony wishes to survive), and thus provides varroa with unlimited definitive hosts—meaning that varroa can reproduce rampantly in the hive at any time that the bees are rearing brood. And that’s the crux of The Varroa Problem.
Emerging Parasites
In a natural population of A. mellifera, once this virulent “Korean” strain of varroa is introduced, one of four things will happen:
- The mite will overwhelm the bees and extirpate the population [7]. This occurred on Santa Cruz Island [8].
- The bee population is genetically diverse enough that a fair proportion of colonies are preadapted for resistance. The mites will quickly eliminate susceptible genetic lines, and the remaining bees come into balance with the mite. This occurred in South Africa in m. scutellata and A.m. capensis [9].
- Traits for resistance are rare in the bee population, and the population is reduced to a few surviving colonies exhibiting some degree of resistance. Due to lack of mite transmission between colonies (as with the squirrels), mite pressure is reduced, allowing the most successful colonies to swarm, and thus reestablish a bee population in equilibrium with the mite. This appears to be occurring in feral populations all over the world.
- The bees will completely out-compete the parasite, and varroa will be extirpated. There is no biological reason to expect this to happen. Our bees are stuck with varroa.
In North America, Scenario #2 has occurred in the Africanized bee population below the 30th parallel; Scenario #3 is occurring in the feral populations northward. In most managed populations of commercial bee stock, Scenario #1 plays out on an annual basis, prevented only by chemical intervention by the beekeepers.
Practical application: we beekeepers, in our efforts to maintain our livelihoods, have inadvertently slowed or prevented the natural process of host/parasite coevolution.
In order to understand why, we need to understand both the symbiotic relationship between varroa and DWV, as well as how they are affected by different routes of parasite transmission.
The Varroa/Virus Complex
The Varroa Problem is actually more of a virus problem, since a colony typically dies from an epidemic of one or more varroa-transmitted viruses long before it would succumb from the impact of the mite itself [10]. This concept was clearly elucidated by Drs. Sumpter and Martin in 2004 [11]. Dr. Martin subsequently showed how Deformed Wing Virus (DWV) quickly adapted to take advantage of the vectoring ability of varroa [12].
It just keeps getting more interesting. Not only did DWV evolve from being an occasional parasite in bee hives (to become a ubiquitous infection in varroa-infested colonies), but it appears that in most hives one virulent strain tends to outcompete all others [13]. And varroa and DWV have apparently formed a mutualistic symbiotic relationship. In this relationship varroa benefits the virus by acting not only as an in-hive vector and promotor of the virus’s ability to surmount the bees’ immune responses, but also as a highly-efficient vector to transport the most virulent strains of the virus across substantial distances to infect other hives.
The virus in turn helps the mite. Di Prisco [14] recently found that not only does DWV suppress the bee’s immune system overall, but that a severe DWV infection of the pupae in turn benefits varroa, increasing the proportion of foundresses that produce offspring [15]. DWV also goes directly to the bee’s brain [16], where it likely affects bee behavior, perhaps resulting in increased drift of affected bees to other hives [17]. Thus, both the mite’s bloodline, as well as that particular strain of virus, would be transmitted to a new hive. But most importantly, the delayed epidemic of DWV in a mite-infested colony allows the mite population to build up to huge levels by late summer or fall. And at that time, DWV suddenly weakens the honey-filled hive during the nectar dearth. Robbing bees (easily identified by the mites by their different colony odor) then serve as taxi service for those mite bloodlines that have produced the most offspring.
Practical application: some have proposed that an “avirulent” mite may evolve. This could only occur in a reproductively-isolated, vertically-transmitted population. In colony-dense managed apiaries, those mites that are most successful at reproduction will always outcompete any others. This is exactly what happened in South America (apparently even in the feral population), where the more virulent Korean strain of varroa has replaced the initial and “gentler” Japan strain.
It May Get Worse Before It Gets Better
It’s becoming clear that DWV can rapidly evolve in response to opportunity [18]. Those of us who’ve kept bees since the beginning of the varroa invasion have watched DWV become more and more virulent. And it’s no longer clear whether DWV is a single “quasispecies,” or whether hybridization between two different viruses is going on, creating novel strains of even greater virulence (reviewed in [19]). In any case, due to the deadly combination of viral evolution, current beekeeping practices, and the inevitable loss of efficacy of miticides, it’s likely that the varroa/virus complex is going to become even more of a problem before it gets better.
Practical application: people resist any sort of change until it simply becomes too painful to continue doing things “the old way.” My hope is that the expected worsening of The Varroa Problem will finally kick start us into adopting the long-term solution—better breeding.
Next I’ll return to the biology of parasite transmission…
Acknowledgements
Thanks to all the scientists who have done the research upon which this article is based. And to those Early Innovators who have demonstrated that treatment-free beekeeping is possible. And as always, my thanks to Pete Borst for his help in research.
Notes and Citations
[1] Although many of the colonies that I sell to hobbyists manage to survive for some years without treatment (this is not a sales pitch—I’m not looking for more customers).
[2] Pen and ink drawing of the follicle mite (Demodex folliculorum) by A.J.E. Terzi, ca. 1919. Image of woman’s face (Homo sapiens), Wikimedia Creative Commons.
[3] This is a very important point—the host (the bees) set the rules. I’ll delineate the rules set by Apis cerana later in this series.
[4] Solignac, M, et al (2005) The invasive Korea and Japan types of Varroa destructor, ectoparasitic mites of the Western honeybee (Apis mellifera), are two partly isolated clones. Proc. R. Soc. B 272: 411–419.
[5] Rath, W (1999) Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie 30: 97-110.
[6] Likely olfactory.
[7] An introduced parasite can certainly drive a host to extinction—think of the American Chestnut and introduced blight fungus, the Hawaiian birds and the malaria parasite, or the many amphibian species that have gone extinct due to the introduction of the chitrid fungus.
[8] http://iws.org/CISProceedings/7th_CIS_Proceedings/Wenner.pdf
[9] Allsopp, M (2006) Analysis of Varroa destructor infestation of Southern African honey bee populations. http://www.repository.up.ac.za/dspace/bitstream/handle/2263/27094/dissertation.pdf?sequence=1
[10] There is the exceptional colony that exhibits strong resistance to viruses, and can tolerate incredibly high varroa levels. See Fig. 5 in, Genersch, E, et al (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie DOI: 10.1051/apido/2010014
[11] S u m p t e r, DJT & SJ M a r t i n (2004) The dynamics of virus epidemics in Varroa-infested honey bee colonies. Journal of Animal Ecology 73: 51– 63.
[12] Martin, SJ, et al (2012) Global honey bee viral landscape altered by a parasitic mite. Science 336: 1304-1306.
[13] Mordecai, GJ, et al (2015) Superinfection exclusion and the long-term survival of honey bees in Varroa-infested colonies. The ISME Journal 1 – 10.
[14] Di Prisco, G, et al (2016) A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. PNAS 113 (12): 3203–3208. Open access.
[15] Likely by suppressing the pupa’s immune response at the feeding wound.
[16] Shah, KS (2009) Localization of deformed wing virus (DWV) in the brains of Apis mellifera (European Honey Bees). Honor’s Theses. Paper 19. Bucknell University.
[17] It is common for parasites to change the behavior of their hosts to better effect transmission (this is why cold viruses make you sneeze). DWV has been shown to go directly to the bee brain, and to cause premature exit of infected bees from the hive, as well as affecting their orientation. I have not yet seen firm evidence that DWV increases drifting of bees to other hives, but I strongly suspect that it does. Further information at:
Li, Z, et al. (2013) Viral infection affects sucrose responsiveness and homing ability of forager honey bees, Apis mellifera L. PLoS ONE 8(10): e77354. doi:10.1371/journal.pone.0077354
Annoscia, D, et al (2015) Mite infestation during development alters the in-hive behaviour of adult honeybees. Apidologie 46(3): 306-314. Open access.
[18] Martin, SJ, et al (2012) op cit.
[19] McMahon DP, et al (2016) Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. R. Soc. B 283: 20160811.
A quote from the above: “our findings indicate that DWV-B replication in A. mellifera may in fact be superior to that of DWV-A. Regardless of any uncertainty over the putative adaptive origins of DWV-A and -B, the potential for novel strains with significantly altered virulence dynamics to emerge via recombination should be acknowledged.”
[20] (added post publication): Martin, S, et al (1997) Non-reproduction in the honeybee mite Varroa jacobsoni. Experimental & Applied Acarology 21: 539–54. Martin cites reports of A. mellifera colonies coexisting next to A. cerana colonies for many years without collapsing.




