Understanding Colony Buildup and Decline: Part 13b – The Winter Continued
Understanding Colony Buildup and Decline: Part 13b
The Winter Continued
Randy Oliver
ScientificBeekeeping.com
First published in ABJ August 2016
In the previous article I summarized the basic mechanics and physics of the winter cluster. But I still had some “leftovers” worthy of further discussion…
The Optimal Size of the Winter Cluster
I showed in Part 12 of this series [1] how colonies appeared to gravitate towards a 9-10–frame winter cluster. This makes me curious as to whether it is incidental that swarms prefer cavities that would just hold that many bees. Is there something about that size cluster that is to the bees’ advantage?
Farrar [2] advised wintering very large colonies in the northern states, containing “8 to 10 pounds of bees that emerged after August 20.” He may not have understood that it was the last-emerging workers that would become long-lived “winter bees,” but clearly understood the importance of broodrearing in fall.
Scientific note: here we have a problem of quantifying the strength of a colony in cold weather. The most accurate practical method (as opposed to counting thousands of bees one by one) is to first weigh the hive, then shake all the bees from it and weigh it again. By subtraction, one obtains the weight of the bees alone. Depending upon how much food there is in their bodies, there are roughly 4000 bees per pound. Thus, Farrar suggested that the ideal colony would go into winter with 32-40,000 bees. This is as large as the peak summer population of hives in most parts of the world, which brings up the interesting observation that colonies tend to grow larger in the more northerly areas of North America than they do further south [3]. I have put quite a bit of time into trying to figure out why [4], and suspect that it is largely due to better forage opportunities.
It’s more difficult to quantify colony strength by eye. By actual count, there are generally 1800-2400 workers on a fully-covered comb in warm weather. When I shake packages, 2 fully-covered combs typically yield about a pound of bees. So for general purposes, 2000 bees per comb in warm weather is a decent rule of thumb. But when it gets cold, those same bees can cram themselves into a much smaller cluster.
In my articles, in order to keep the reader’s head from swimming with numbers, I prefer to quantify colony size by the accepted standard for almond grading—the number of deep Langstroth combs at least 70% covered by bees at a temperature above 60˚F. This would again be in the ballpark of 2000 bees per frame. Thus, Farrar’s 40,000-bee colony would completely fill at least a double deep in warm weather, since there are typically few bees over combs of sealed brood. He’s talking about going into winter strong!
Others have questioned Farrar’s recommendations, at least as far as for other regions. Jeffree and Allen [5] point out that “most authors give the impression that increased colony size is a decided advantage” for overwintering colonies. However, their research in Aberdeen, Scotland suggested that for unsupplemented colonies, that there were “optimum” sizes for minimizing the proportional loss of bees over winter. Their data indicated that optimum colony size in would be 13,000 bees in October for colonies free of nosema, and 16,000 bees for those infected by the parasite (that works out to around 6.5 or 8 frames covered with bees in October (on a warm day), respectively).
Practical application: I cannot recommend Farrar’s excellent bulletin highly enough—it is required reading for any serious beekeeper. In the cold-winter, late-spring, clover-rich region of Wisconsin in which he performed his research, there’s only 7-8 weeks from first tree pollens ‘til the main honey flow, which would then last for a month (followed later by a fall nectar and pollen flow). Since it takes 5-6 weeks for a strong overwintered colony (containing 5-8 frames of brood) to reach maximum strength, he needed colonies to come out of winter already strong.
In other areas, there may be different timing. For example, where I live, we may get two minor, and one major honey flow (plus a later honeydew flow): the first at 7 weeks after first tree pollen (this flow is often rained out), at 12 weeks (again often suppressed by weather), the main flow at 18 weeks, and honey dew at 28 weeks (but little incoming pollen after 18 weeks, and only the most minor fall nectar flow). Compared to Wisconsin, where a strong colony could make a few hundred pounds of honey on the main flow alone, I am lucky to make 50 pounds of surplus overall. But because I am an almond pollinator, my colonies need to be strong mid February, meaning that my main problem in spring will be swarm prevention.
Thus, optimum management is region (and income source) specific, and as beekeeper Bill Truesdell repeatedly reminds us on Bee-L, “all beekeeping is local.”
Bernhart Möbus followed up on Jeffree’s work, and later wrote some lively articles in ABJ. Möbus offered bee management recommendations that were, in his words “based on biological investigations rather than on traditional theories which have only gained credence by being repeated frequently—and slavishly—in most books.” He pointed out [6] that the results of his and Jeffree’s experiments showed that “any large deviation from the ideal size [of a wintering colony]—up or down—was ‘punished’ by Nature with occasionally heavy losses of bee life.”
Möbus also experimented by creating supersized colonies in fall (by combining), and found that such colonies didn’t rear brood during winter, and foraged desperately for water. He explained these observations by the physics of water balance: bees in the insulating shell would be awash in metabolic water due their heavy consumption of honey coupled with their cool body temperature. On the other hand, any bees in the warm core would be thirsty as heck. He thus made the case that “when there are too many bees in a hive, or when the hive is too insulated and too warm for a mild climate, thirst-crazy bees were driven to fly out at the slightest excuse; not to gambol in the sunshine, but to collect water.”
His supersized colonies suffered from disastrous winter losses. Not only were there water balance issues, but he had an explanation that I found intriguing:
The general finding that very large colonies suffer heavy loss equally with very small colonies is surprising, and no data are here brought forward to indicate the cause of this clear effect. It is, however, evident from the mathematics of heat distribution and transference within the cluster that as the cluster size increases the central temperature necessarily becomes higher for any given value of the surface temperature. It is therefore, suggested as a probable explanation that in a very large cluster the bees have some difficulty in keeping the center of the cluster cool enough while at the same time retaining a sufficiently high surface temperature.
Practical application: I can perhaps reconcile Farrar’s and Möbus’ contradictory findings by the fact that the winters in Wisconsin are far colder than those at Aberdeen. Thus, excessive heat would not be a problem in the colder climate.
Free [7] found that a greater proportion of bees appeared to survive over winter in larger colonies, but that the benefit seemed to level off at around 18,000 bees (roughly a 9-frame cluster on a warm day).
Practical application: the observations of various researchers, coupled with those of my own (as an almond pollinator) suggest that there are likely optimal cluster sizes for wintering colonies . That size would depend upon the severity of cold that the colony will face, the duration of the period in which there is no flight or foraging, and the beekeeper’s desired size of the colony in early spring (based upon pollination needs, swarm avoidance, desire to make increase, or the timing of honey flows).
Of interest is that in cold-winter climes, Nature has selected for bee races that form relatively small, frugal winter clusters; whereas in in Mediterranean regions larger clusters are the norm. Beekeepers, who typically keep bees of mixed heritage, additionally have the option of managing colony size by supplemental feeding. Such feeding should be done only with an understanding of the resultant consequences (e.g., early feeding may result in excessive swarming or late-spring starvation).
Honey Consumption and Winter Broodrearing
In my area, colony weight gain is often over by early July, after which there are no significant sources of nectar until mid February. That means that for a colony to survive without my help, that it must live on little more than those stores for seven months of the year.
The winter cluster is remarkably efficient at conserving energy, and thus minimizing its consumption of precious honey stores. As Möbus [8] colorfully points out, “taking the strength of a ‘normal’ winter colony as between 10,000 to 15,000 bees, the average consumption of honey per bee and per day under insulated conditions was so minute (1.0-1.5 mg/day/bee) as to make us wonder if the bees lived indeed on love and altruism, rather than on honey.”
Ideally, the cluster initially forms at the bottom of the hive, and then works its way upward through its honey stores during the winter. But occasionally things don’t work out, as explained by Johansson [9]:
During moderating temperatures, the cluster will break up. With the return of a sudden cold period, the bees re-cluster and by chance may choose a location not well provisioned with food. Some winter losses are of the coincidental nature, and may involve large colonies that were left with ample stores. The colony will be found dead in the spring, with honey stores at some distance from the cluster of dead bees.
Practical application: successful beekeepers are generous in leaving their colonies with plenty of honey above the cluster, feeding syrup, if necessary, prior to cold weather so that the colony can best arrange its stores. As Farrar points out (and as I’ve noticed), having a central frame in the upper box with a low patch of empty brood comb at the bottom (to bridge the gap between the brood chambers if the upper box is chock full of honey) helps the cluster to move upward when necessary.
Initiation of Winter Broodrearing
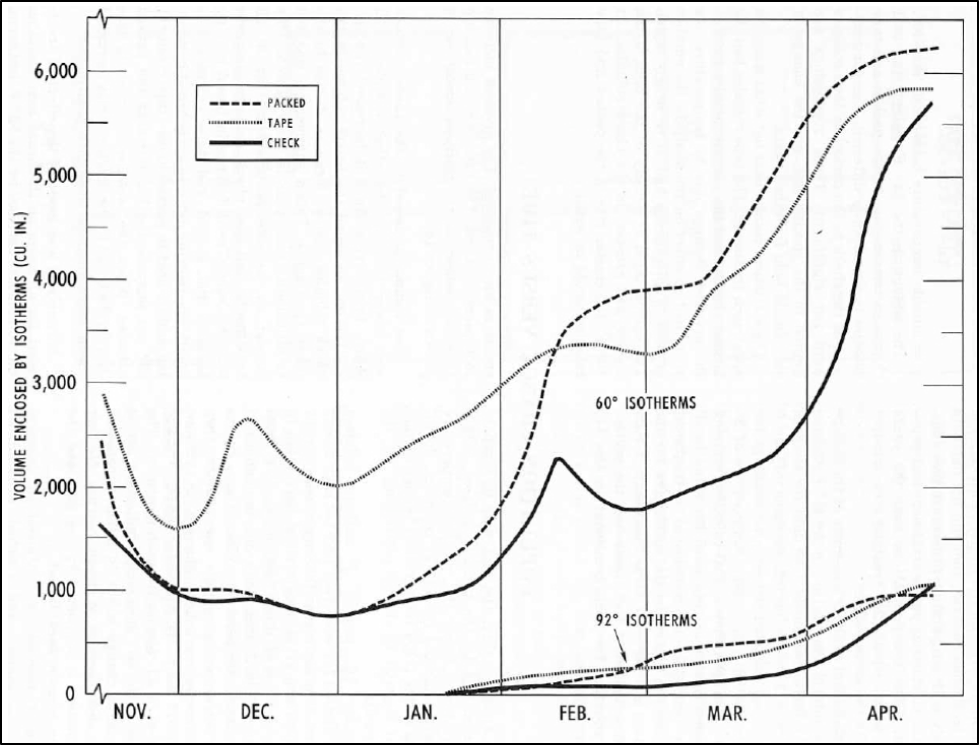
Colonies typically initiate broodrearing in midwinter, as illustrated by Owens (Fig. 1), typically in December or January.
Figure 1. Note how the colony expands the cluster size (upper plots) once it commences broodrearing. The appearance of the 92˚F isotherms (lower plots) indicate that the cluster has brought its core up to broodrearing temperature. Note how the volume of that broodnest then expands into spring. The combination of warming temperatures and an expanding broodnest causes the cluster to expand dramatically (uppper solid dark line). Figure from Owens [[i]] (the Check hives were unprotected, Packed were insulated, and Tape were electrically heated to 40˚F).
[i] Owens, CE (1971) The thermology of wintering honey bee colonies. USDA Technical Bulletin 1429.
Such winter broodrearing is necessary to replace workers that have died over winter, and to start building the colony population in anticipation of early spring forage. However, the rearing of brood and the heating of an enlarging broodnest greatly accelerates the consumption of winter stores. Free [11] found that smaller colonies devoted more of their resources to broodrearing, and thus consumed more honey per bee.
By periodically opening and inspecting some 47 hives over the course of the Aberdeen winter (freezing temps, but not extreme cold), Möbus found that honey consumption was directly correlated with the amount of broodrearing. Colonies that reared little brood between November and February consumed less than 10 lbs of honey. Those that reared more brood could double their honey consumption (jumping ahead, the rate of honey consumption really picks up during the spring turnover and linear growth phase, when even strong colonies can starve during a brief bout of foul weather [12].
Practical application: the above is often learned the hard way by those pollinating almonds in Northern California, where it often rains during almond bloom. Many a time I’ve needed to take emergency feeding measures (or swapping of honey combs) to prevent my strongest colonies from starving during a winter storm while they are in the orchards. Sometimes we’ve run to the nearest grocery and returned with sacks of granulated sugar to dump over the top bars, and once saved a colony (in which the bees were too hungry to even move) with a can of soda pop.
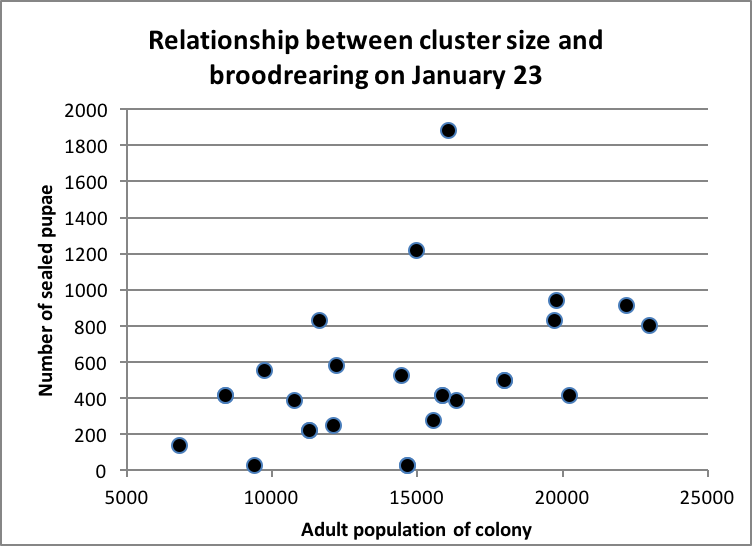
The smaller the cluster, the smaller the broodnest it is able to maintain. I analyzed Lloyd Harris’ data set to see whether this held true for his shed-wintered colonies–it did (Fig. 2).
Figure 2. Not surprisingly, there was a general trend for larger colonies to rear a greater amount of brood in midwinter. One of Harris’ shed-wintered colonies actually came out of winter with a larger population than it started with! Data courtesy Lloyd Harris.
Practical application: beekeepers with almond pollination contracts, as they desperately attempt to build the strength of their hives prior to grading, are acutely and frustratingly aware that small colonies in midwinter are slow to grow. A colony can only rear as much brood as it can keep warm. No matter how much you feed and pray, you simply can’t circumvent the laws of physics.
Pollen Consumption
Farrar made a huge point of the requirement for serious pollen stores (in the form of beebread) in the hive in order for a colony to be able to maintain broodrearing in late winter. But let me share something that I observed when I was crushing worker bee samples to track the fate of consumed pollen sub (labeled with fluorescent pigment) [13]. During the summer, only a fraction of the workers—the nurse bees—had any pollen in their guts. But to my surprise, when I took samples in November every danged worker was chock full of pollen (Fig. 3).
Figure 3. In this sample of 50 bees, taken from the outer frame of the upper hive body on November 7, following 2 days of confinement due to rain, every single bee had a gut full of pollen.
Citizen science opportunity: I’ve started a number of times to take weekly samples of bees from a colony in order to track how the percentage of bees with pollen in their guts changes over the course of the season, but have failed each time to follow through. I’d appreciate hearing from volunteers who’d like to collect such data. Please contact me for instructions.
Anyway, as pointed out by Dr. Kirk Anderson, the most secure place for bees to store pollen may be within their bodies. Interestingly, the pollen in the guts of the bees above was mostly in their hindguts (rectums), and consisted largely of pollen exines from which the innards had already been digested in the midgut. My question then is whether what I observed was simply the result of all the bees in the hive consisting of “winter bees” that were temporarily gorging on whatever pollen they could consume, or whether there is nutritional benefit to the bees from holding digested pollen in their hindguts for an extended period of time.
Scientific note: we still have a great deal to learn about bee biology!
In any case, beebread stored in fall is critical for colony buildup in late winter and early spring, prior to the shedding of the first tree pollens. In the East, goldenrod and aster pollen serves the purpose; in the West, Coyote Bush and Rabbitbrush. Without such fall pollen stores, colonies simply cannot engage in late-winter broodrearing.
Practical application: in the arid West, many of us find great benefit from feeding pollen sub in late summer/fall, and perhaps again in January if weather prevents foraging on early pollen sources (I find no benefit if the bees are getting natural pollen).
Cleansing Flights and Dysentery
After bees consume pollen, they gotta dump the undigested waste, and for sanitary reasons they avoid doing it within the hive, instead taking “cleansing flights.” But they can’t do so if cold weather confines them to the hive during winter. Nature has thus provided bees with a highly extensible rectum, which, if you’ve ever stood under the flight path of bees on the first flight day after prolonged confinement, results in a “yellow rain” that can be truly amazing in quantity and aroma.
A number of researchers have brought up the relationship of water balance and rectal contents. Remember, that the physics of relative humidity (as well as experimental evidence) strongly suggest that bees in the insulating shell of the cluster would gain water, whereas those in the heated core would lose water. Add to this that it is the bees in the heated core that would be consuming beebread in order to rear brood. Thus, so long as the cluster is large enough to maintain a heated core, waterlogged bees could theoretically move from the shell to the center of the cluster to lose excess water by evaporation (and vice versa for thirsty interior bees).
As pointed out by Simpson [14]:
Cluster humidity is physiologically important to adult bees in winter as well as to brood at all times. Honey bees do not normally discharge their rectal contents in the nest, and when they are confined to the cluster by cold, they cannot obtain water should they require it, or discharge outside any surplus they accumulate. Forced discharge of rectal contents in the nest (“bee dysentery”) can kill a colony. Surviving long periods of confinement, therefore, depends on a balance between water production from metabolism of food and water loss by evaporation from the bees’ bodies. The balance is made possible by the dryness (15 to 20 percent moisture) of the winter food (honey).
Practical application: hence the common admonition not to feed thin syrup to bees during a cold winter. Honey, fondant, or even dry sugar may be more appropriate.
Cleansing flights may play an important role in successful wintering of colonies (a problem with shed wintering).
Practical application: a number of beekeepers prefer only minimal wrapping of hives in cold-winter areas, since thick insulation prevents temporary warming of the hive during warm breaks in the weather, which allow both cleansing flights and rearrangement of the cluster over the remaining honey stores. Top insulation and good drainage also help to prevent moisture buildup. Upper entrances may (or may not) be of benefit regarding regulation of humidity, but certainly provide easy egress for cleansing flights and the “altruistic self removal” of senescent or diseased workers.
Unfortunately, things do not always go well for the bees, resulting in some workers not being able to “hold it,”or to hold it only long enough to barely clear the entrance (Fig. 4).
Figure 4. Dysentery is often dramatically displayed at the colony entrance. Although it is apparently not caused by nosema, if fecal contents are discharged within the hive, pathogens would certainly be transmitted. Photo courtesy beekeeper Monique Vescia.
Phillips [15], when measuring temperatures in overwintering colonies, observed that:
Honeydew honey is a poor food for winter and is so recognized. It contains the same sugars as honey, but contains in addition a considerable amount of dextrin [as well as other indigestible complex sugars and perhaps a high mineral content]… From the evidence at hand it appears that dextrin cannot be digested by bees and, whether or not this is the explanation, honeydew honey causes a rapid accumulation of feces which usually results in the condition known as dysentery, in bad cases of which the feces are voided in the hive. In the case of [a colony wintered on honeydew] the whole hive inside and out, as well as the frames and combs, were spotted badly, the inside of the hive being practically covered. Even with fine honey stores such a spotting is usually noticed after a prolonged confinement, especially in severe weather (or during brood rearing). It therefore appears that the accumulation of feces acts as an irritant, causing the bees to become more active and consequently to maintain a higher temperature. We are therefore justified in believing that the cause of poor wintering on honeydew honey is due to excessive activity, resulting in the bees wearing themselves out and ultimately in the death of the colony…
It therefore follows that excessive activity causes the consumption of more food, resulting in turn in more feces, so that colonies on poor stores are traveling in a vicious circle, which, if the feces can not be discharged, results in the death of the colony. In the work here recorded no attention was paid to the theory that dysentery is due to an infection, since there is nothing in the observations made that lends any support to that idea.
Practical application: Recall Möbus’ counsel to beware of beekeeping fallacies being parroted in the literature [16]. I have repeatedly pointed out that there is scant, if any, evidence supporting that Nosema apis causes dysentery, and zero evidence that it is caused by the most prevalent species of nosema found in colonies today—N. ceranae. I’ve viewed dozens of samples of dysentery from all over the country, and see no correlation between dysentery and nosema, other than the obvious fact that if a nosema-infected bee defecates within the hive, it is likely to transmit nosema spores to other bees [17]. So I have a really hard time when beekeepers blame their problems on nosema simply because they see signs of dysentery.
Factors that can cause dysentery are:
- small clusters having trouble with water balance,
- honeydew or poor-quality pollen stores irritating the gut, or causing microbial problems (I often observe yeasts in dysentery samples) [18],
- heavily-feeding nurse bees in spring confined by foul weather,
- certain pollen flows with apparent laxative effect,
- or when the beekeeper has fed poorly-digested or irritating pollen or sugar supplements, which leads us to…
Stimulatory Feeding
As well explained by Farrar, timing of buildup is everything. To that end, many beekeepers engage in stimulatory feeding of their colonies. Of considerable interest, is that Farrar [19] also found that late-winter/spring “’stimulative feeding’ does not stimulate a colony to attain a higher rate of brood-rearing when ample stores are present in the hive.”
I’ve found the same thing—that a well-provisioned colony during its late-winter buildup phase seems to respond to a sugar-rich pollen sub alone, and does not require syrup, although it seems to me (no hard data) that the addition of syrup improves buildup. If colonies are light, then syrup is definitely called for, as colonies without honey reserves appear to “hold back” (again, no hard data). At other times of the season, the full benefit of pollen sub is not realized unless syrup is also fed [20].
Practical applications: an early feeding of syrup midwinter may “wake up” a colony, but can actually harm it if it stimulates “fruitless foraging” with no return of the stimulated foragers’ investment (it just wears out the winter bees without increasing recruitment). Late-winter buildup is far more dependent upon the availability of beebread or pollen sub.
In my area, where colonies often go into winter with the combs packed with beebread consisting of rust fungus spores [21] and dark honeydew honey, colonies greatly benefit from fall feeding of pollen sub and sugar syrup. This also brings up the point that not all aged beebread may be good for bees [22].
Realize that most commercial Italian-type bee stocks are selected to be “All America” winners for all areas, provided that they receive appropriate husbandry. This means that they are selected to respond to supplemental feeding and mite treatment, and to exhibit full-tilt broodrearing whenever stimulated. There is nothing wrong with such selection for this or any domesticated livestock strain. But one must then be careful not to stimulate such bees at the wrong time, as it can lead to swarming or starvation.
It’s also likely that locally-adapted and naturally-selected stocks may use more discretion in which sources of nutrition they store for winter use, or may be better adapted to those food items.
Dang, still not done with the topic of wintering. I’ll continue next month.
Acknowledgements
Thanks to Pete Borst and Lloyd Harris, both of whom go out of their ways to help me. And to Monique Vescia for the photo. And to my new bride, Stephanie (after 15 wonderful years together) for suggestions to the manuscript.
Notes and Citations
[1] See Figure 2 in Part 12—Late summer through autumn.
[2] Farrar, CL (1944) Productive management of honeybee colonies in the northern states. USDA Circular No. 702. Required reading, easy to find on the Web.
[3] As brought to my attention years ago by Dr. Eric Mussen, who also studied bees in Minnesota, and then moved to California.
[4] I’ve plotted out differences in daylight hours, adjusting for actual flight hours after accounting for rain and wind speed, and could not convince myself to accept that simple explanation.
[5] Jeffree, EP & DM Allen (1956) The influence of colony size and of nosema disease on the rate of population loss in honey bee colonies in winter. JEE 49(6): 831 – 834.
[6] Möbus, B (1998) Rethinking our ideas about the winter cluster. Three parts in ABJ 1998 July, Aug and Sept.
[7] Free, JP & PA Racey (1968) The effect of the size of honey bee colonies on food consumption, broodrearing, and the longevity of the bees during winter. Ent. Exp. & Appl. 11: 241–249.
[8] Möbus, B (1998) Part II. ABJ August 1998: 587-591.
[9] Johansson, TSK & MP Johansson (1979) The honeybee colony in winter. Bee World 60:4, 155-170. This is a very nice review.
[10] Owens, CE (1971) The thermology of wintering honey bee colonies. USDA Technical Bulletin 1429.
[11] Free (1968) op cit.
[12] This is often painfully observed by my sons and I during our post-apple-bloom dearth, when a spring weather event may force rapidly-growing splits (fat with honey the week before) into brood cannibalism or major adult kill from starvation.
[13] Sorry, results not yet published.
[14] Simpson, J (1961) Nest climate regulation in honey bee colonies. Science, New Series 133(3461): 1327-1333.
[15] Phillips, EF & GS Demuth (1914) The temperature of the honeybee cluster In winter, Bull. 93, U.S. Dept. Agr.
[16] This is an example of the fallacy of “reification,” by which an attractive and logical explanation is taken as a truth, and then concretized by repetition. Another example would be that since neonics are neurotoxins, and started be to used around the time that beekeepers suffered increased colony losses, that they were obviously responsible for most bee health problems. Then keep repeating that mantra until everyone believes it.
[17] This hypothesis is supported by Moeller, FE (1972) Effects of emerging bees and of winter flights on nosema disease in honeybee colonies. J. Apic. Res. 11(2): 117-120.
[18] Dr. Kirk Anderson is investigating the effect of diet upon the bacterial community in the gut.
[19] In his thesis, of which I only have read excerpts.
[20] Somerville, D (2005) Fat Bees Skinny Bees, A manual on honey bee nutrition for beekeepers. Rural Industries Research and Development Corporation, Australia. http://www.rirdc.gov.au/reports/HBE/05-054.pdf
[21] https://scientificbeekeeping.com/fried-eggs-identified/
https://scientificbeekeeping.com/a-comparative-test-of-the-pollen-sub/
[22] I’m involved in a paper in currently in review, collaborating with Patrick Maes of the Kirk Anderson lab at ARS Tucson