Understanding Colony Buildup and Decline: Part 10- Summer Downsizing and Varroa
Understanding Colony Buildup and Decline: Part 10
Summer Downsizing And Varroa
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in March 2016
At this point in time, the desperate intensity of spring buildup has long since passed, swarming season is over, and the colony has had its chance to use the main honey flow to put on winter stores. It’s now difficult to speak generically, since, depending upon latitude and environment, it may be only weeks before the onset of winter, or there may yet be a slow trickle of nectar and pollen coming in for months. Or there could be a late-summer lull, followed by an intense fall nectar and pollen flow. And in the arid West, the colony may be facing a long, dry dearth until the first fall rains.
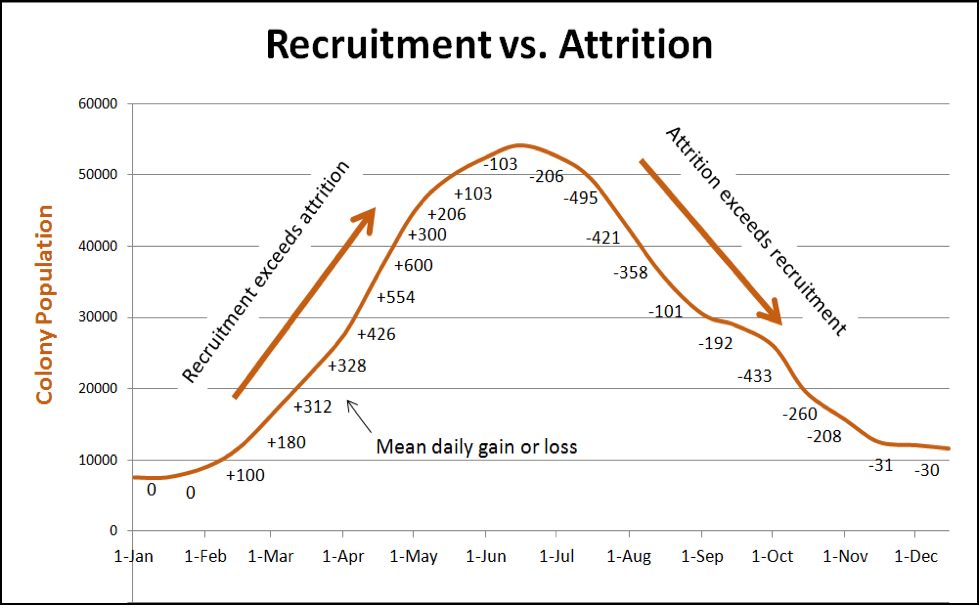
In any case, colony population typically peaks during the main flow. Then, depending upon summer forage availability, temperature, and rainfall, attrition will exceed recruitment (meaning that more workers die each day than emerge from the broodnest—see Fig. 1), and the population declines. Unless there is a fair amount of pollen and nectar coming in, the colony will shift at some point to conservation mode in order to conserve stores and prepare for winter (in this article I’ll stick to colony dynamics in areas without winter bloom [1]).
 Practical application: lots of bees die through normal attrition every day. The beekeeper doesn’t normally notice it since those bees die in the field (including those that do so due to altruistic self removal). Since this high rate of attrition is normal, in order to maintain colony strength, uninterrupted broodrearing is critical. And that requires a steady supply of pollen.
Practical application: lots of bees die through normal attrition every day. The beekeeper doesn’t normally notice it since those bees die in the field (including those that do so due to altruistic self removal). Since this high rate of attrition is normal, in order to maintain colony strength, uninterrupted broodrearing is critical. And that requires a steady supply of pollen.
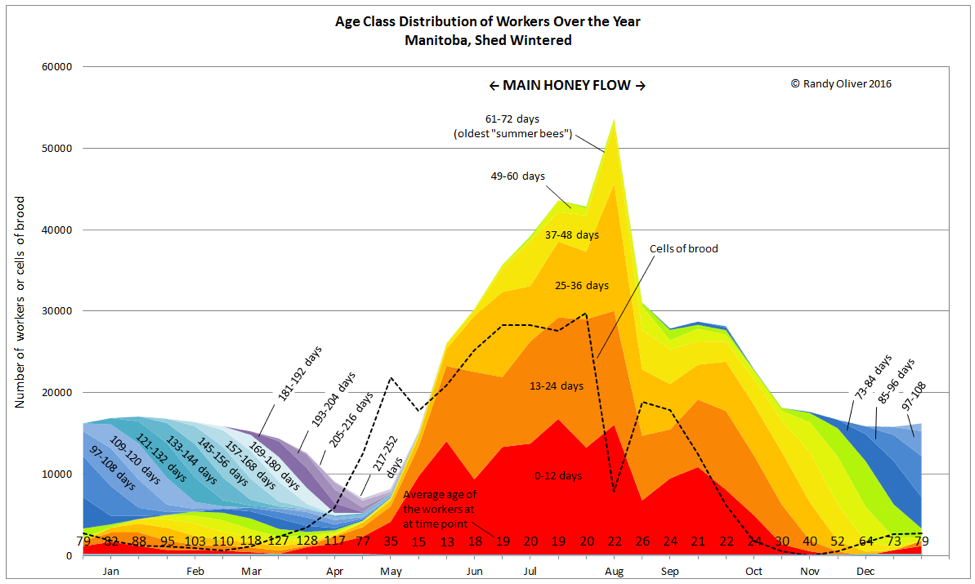
The above graph is for illustrative purposes only—bees of course love to remind us that there are exceptions to every rule. Colony dynamics may vary greatly from location to location, and season to season, and the population curve under real world conditions may exhibit “hiccups” due to any number of factors (as in Fig. 2). In any case, whenever the attrition of workers exceeds recruitment, the colony population will decline. This occurs whenever there is not enough incoming pollen to sustain serious broodrearing.
Figure 2. We’ve now passed the main flow. In this case, it was so intense that the bees plugged out the broodnest with nectar, temporarily restricting the queen’s egg laying (resulting in a sharp drop in population due to the break). She was able to rebound for a short period in September, then as fall progressed, broodrearing was curtailed, and the population further declined in preparation for winter [2].Up ‘til now, the high rate of recruitment (broodrearing) has sustained a vibrant population of young workers (see the average worker age along the bottom of the chart)—as with other species, a rapidly-growing population consists largely of young individuals. On the other hand, a declining population starts to lose its youthfulness and resiliency.
The Need For Pollen
Honey bees can survive in an incredible diversity of landscapes. The population dynamics of the colony reflect the abundance of resources. In general, colonies respond initially to the first tree pollens, then build rapidly on early spring nectar and pollen sources, and reach peak population during the early- to midsummer main flow or flows. After that, the truism “all beekeeping is local” applies. There are areas in which moisture, temperature, and local vegetation supply forage for the rest of the summer, but more commonly, there’s a late-summer pollen dearth (Fig. 3).
 When pollen is scarce, every bee in the colony soon knows it, due to a reduction in the colony’s currency of protein—the precious jelly produced solely by the nurses. The beekeeper can immediately recognize this in the broodnest. The band of reserve beebread disappears, and the brood pattern becomes spotty (Fig. 4).
When pollen is scarce, every bee in the colony soon knows it, due to a reduction in the colony’s currency of protein—the precious jelly produced solely by the nurses. The beekeeper can immediately recognize this in the broodnest. The band of reserve beebread disappears, and the brood pattern becomes spotty (Fig. 4).
 There is nothing necessarily wrong with this late summer decline. As I showed in my last article, it is actually critical, in order to conserve honey stores for the winter. But it also puts the colony under stress. The aging, protein-deficient population loses its resiliency, and is less able to deal with parasites, pathogens, and pesticides (including beekeeper-applied miticides) [3].
There is nothing necessarily wrong with this late summer decline. As I showed in my last article, it is actually critical, in order to conserve honey stores for the winter. But it also puts the colony under stress. The aging, protein-deficient population loses its resiliency, and is less able to deal with parasites, pathogens, and pesticides (including beekeeper-applied miticides) [3].
During our August pollen dearth, our brood frames often look that that in the photo above. When I look closely, I often see that the queen is still laying plenty of eggs, but that there are no young larvae, since the nurse bees, being short on nutrition, simply follow the queen and consume those eggs in order to recycle the protein. In the case of more environmentally-responsive bees like the Russians, the colonies in my yards often went completely broodless during August, and settled into the diutinus state until an autumn pollen flow occurred, at which point they would immediately brood up vigorously.
Practical application: the shrinking of the broodnest and the overall colony population in late summer is a completely natural response by the colony to the changing environment. Locally adapted stock may require no additional husbandry. On the other hand, Italian-type stocks bred for full-tilt production may not respond appropriately, and go into severe nutritional stress.
In the arid West with which I am familiar, during late summer, colonies may shrink in strength to the degree that they may not be strong enough for almond pollination come February. To provide better nutrition, professional beekeepers may move their hives to irrigated land, next to residential areas, or to states where it rains during the summer. Or they may feed supplemental protein (Fig. 5).
 Practical application: feeding an average of about a pound of high-quality protein supplement per week will maintain colony strength; feeding more may allow it to grow (sometimes at a remarkable rate).
Practical application: feeding an average of about a pound of high-quality protein supplement per week will maintain colony strength; feeding more may allow it to grow (sometimes at a remarkable rate).
Recent research [4] has confirmed that (varroa aside) the most important factor for colony health is a continual supply of nutritious pollens. Such a supply depends upon the sort of floral diversity often found in undisturbed habitats, but may also occur in some agricultural and residential areas. The benefit of a steady supply of nectar and pollen may outweigh the harm from agricultural pesticides [5].
Note: before I get jumped on, pesticides can clearly be an issue for colony health in some agricultural areas and instances. Simple enforcement of existing pesticide regulations at the state level would often help.
Lack of protein not only causes a restriction in broodrearing, but also affects the health and longevity of the workers. Nutritional stress hurts the developing larvae, which require constant feeding for optimal development. Poorly-fed larvae grow into sickly, short-lived, virus-susceptible adults. Workers that upon emergence, do not find adequate stores of beebread upon which to feed, are unable to realize full development of their physiology, and due to their short survivorship, exacerbate the decline in colony strength [6].
Practical application: The baseline factor for colony health is the availability of nutritious pollen. Providing protein (either by moving to better pasture or by feeding supplement) is a management tool that allows the beekeeper to adjust colony strength, and perhaps more importantly, to rejuvenate the colony’s workforce. Feeding of enough pollen sub not only restores the flow of jelly in the hive (allowing workers to live longer), but also initiates renewed broodrearing, and thus recruitment of replacement workers.
The Queen’s Last Hurrah
Following the intensity of egg laying earlier in the season, queens in their second year (or older) often peter out.
Practical applications: unlike humans, in which females get better with age (hear that, honey?), queen bees generally don’t. It’s a common misconception that the colony revolves around its queen. In truth, the workers treat the queen as the fungible ovary of the colony—should the queen show any sign of failure, or the colony start performing poorly, the workers will replace her at the drop of a hat.
Annual requeening is one of the best ways to keep your colonies healthy and productive. If opportunity presents after the main flow, this is a good time for requeening, as this is when colonies tend to naturally supersede worn queens in order to establish a vigorous young queen prior to winter. A number of beekeepers report good success in simply placing a protected queen cell up away from the broodnest. Requeening at this time is also a great opportunity for mite control—a subject that I will address in an upcoming article.
Beekeepers Don’t Just Keep Bees
We must also keep in mind that we not only keep bees, but we are also all engaged in providing food for the varroa mite (at times it seems that I’m a better varroa farmer than I am a beekeeper). The food for varroa is bee brood. Thus, this puts us into a quandary regarding management decisions. The highly productive and gentle Italian-type stocks so favored by beekeepers are also loved by varroa, due to the extravagant broodnests that they maintain throughout the season. And managing colonies to be strong for almonds adds to the problem.
Practical application: huge broodnests make for strong colonies. But they also constitute unlimited fodder for varroa—the more brood, the more successfully varroa can reproduce. Thus, feeding colonies is a two-edged sword, as it prevents the colony from starving out varroa during time of pollen dearth (a strategy well exhibited by Russian bees).
A Scientific Note
We Americans need to remember that varroa arrived in Europe a decade before it invaded the U.S. and likewise shortly afterward started wreaking havoc. We must also keep in mind that there are a lot of very sharp researchers in other countries, from which we can learn a great deal. My point—the Europeans have a 10-year jump on us as far as experience with varroa [7], and are a crystal ball for our future.
After varroa first invades, its effects upon beekeeping follow the same trajectory in most every country in which European bees are kept. The first few years aren’t too rough, and a yearly mite treatment does the trick. Then the viruses evolve to take advantage of the mite, and beekeeping gets tougher. Varroa soon develops resistance to synthetic miticides one by one, and beekeepers are forced to shift to organic acids and thymol. And when the beekeepers finally realize that varroa is winning, researchers revisit mite biology in order to try to figure out “biological” controls—see Nazzi’s [8] excellent recent review on that subject. Eventually, we will finally resign ourselves to the fact that the long-term solution is not eve-increasing chemical treatment, but rather in breeding naturally mite-resistant stock. This will require an overall genetic shift of our commercial bee stocks to mite and virus resistant bees.
Practical application: we can see the future—so what the heck are we waiting for?
Anyway, the heyday of varroa research was from the mid 1980’s through around the turn of the century. After that our focus was diverted by CCD and pesticides. Meanwhile, varroa kept chugging along, and researchers (and a number of beekeepers) are now realizing that varroa is indeed honey bee Enemy #1. And if you want to defeat an enemy, you’d better learn all you can about it. So let me explain a bit of mite biology.
Varroa Reproduction
A key thing to keep in mind is that having a few mites in a hive is not a problem. Even 5000 mites placed in a hive would not be a serious problem, so long as those mites did not reproduce, since they would all eventually be groomed off or die of old age. So in order to understand varroa, we must focus upon its Achilles’ heel—its success at reproduction, which defines the mite’s intrinsic rate of increase (r). In order to see the effect of even a minimal 10% decrease in the mites’ r value, I ran a simulation (Fig. 6).
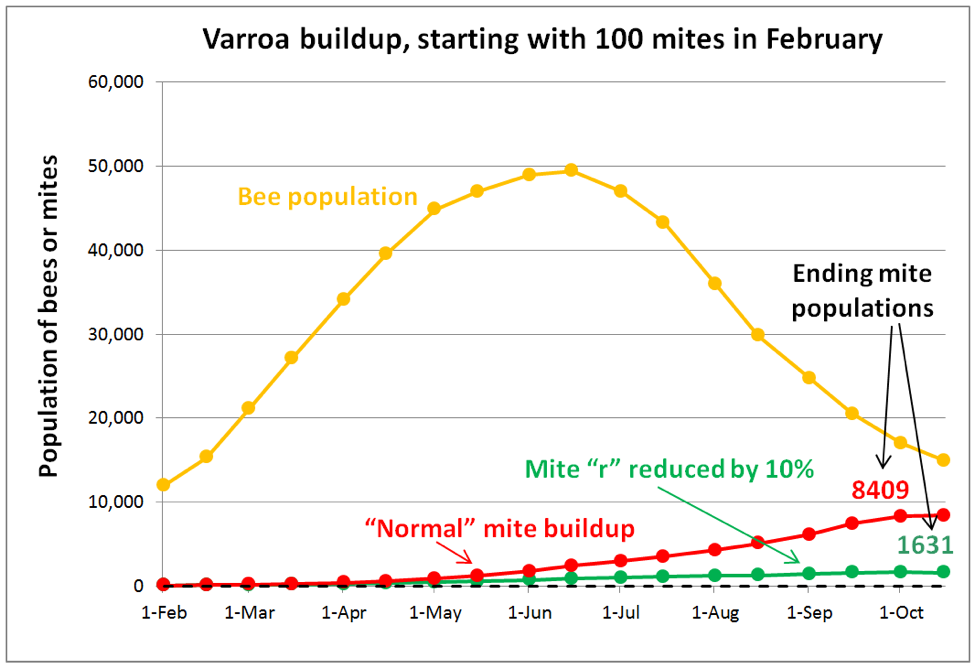 Practical application: in the long term, we are likely going to focus less upon killing varroa, and more upon slowing its success at reproduction.
Practical application: in the long term, we are likely going to focus less upon killing varroa, and more upon slowing its success at reproduction.
The varroa level in a hive is usually at its lowest point when it is first established, or at the spring turnover. The mite population then climbs so long as the bees are rearing brood. The longer that the colony is broodless (during winter, or due to lack of forage), the less time that varroa has to reproduce in a season (this is why varroa management is easier in Canada than in California). The longer the broodrearing season, the more that varroa becomes a problem.
Practical application: the rate of increase of varroa is highest when there’s a full broodnest (especially one rich in drone brood). Unfortunately, managing colonies for optimal income plays into the mite’s hand. When we encourage late-season and midwinter buildup of our colonies for almond pollination, we extend the length of time that the colony maintains a large broodnest (read that, “varroa nursery”). As an almond pollinator, I’m not saying that this is wrong, I’m just saying be aware of the consequences, and deal with them proactively.
A Bit of Varroa Biology
When I perused the varroa research literature for measured rates of mite buildup (with which to build my models), I was at first surprised by the great variation in estimated rates of increase (r values). Then I noticed a pattern—mites build up at a much faster rate when colonies have a high ratio of brood to bees. Upon further research, I found a series of extraordinary papers by Willem Boot and his collaborators (guided by Joop Beetsma) from the Netherlands (published in the mid 1990’s). These guys performed a number of elegant and meticulous experiments on mite invasion into brood [11]. The group’s papers are required reading for anyone seriously interested in mite reproductive success; but I’ll make an attempt at summarizing some of their findings.
A mite is blind, and upon its emergence from the safety of a sealed brood cell, is completely dependent upon its sense of smell in order to find its way first onto a nurse bee (the only bees that will bring the mite into very close proximity to larvae), and then to identify a larvae of the right age for invasion—one preparing to pupate [12]. Olfactory cues given off by the larva at that age triggers the mite to climb off the bee and into the cell.
The point of this is that in a hive, the number of brood cells of the right age on any day is set by the number of eggs that the queen laid 8–9 days previously. At the peak of broodrearing, that can be up to 2000 potential host cells in a hive on any day; during a pollen dearth it may be only a few dozen. In order to locate such a cell, a phoretic mite is largely dependent upon chance for the bee upon which she is hitchhiking to come into close proximity to such a cell (it’s to the mite’s advantage that larvae need to be fed copiously just prior to pupation). Thus, the reproductive success of a mite is largely dependent upon the odds of its ride sticking her head into a cell containing an 8-day larva.
But it’s not that simple. Some mites catch a ride (at least temporarily) on a bee that isn’t a nurse. And only a proportion of nurses are in the broodnest at any time [13], and even then may be tending larvae too young for invasion. What Boot found was the mite success at invasion of a brood cell was directly correlated with the ratio of brood cells suitable for invasion, to the total population of adult bees in the hive. I’ve illustrated how this ratio changes over the course of the season (Fig. 7), using the same brood and adult bee data used to produce Fig. 2 [14].
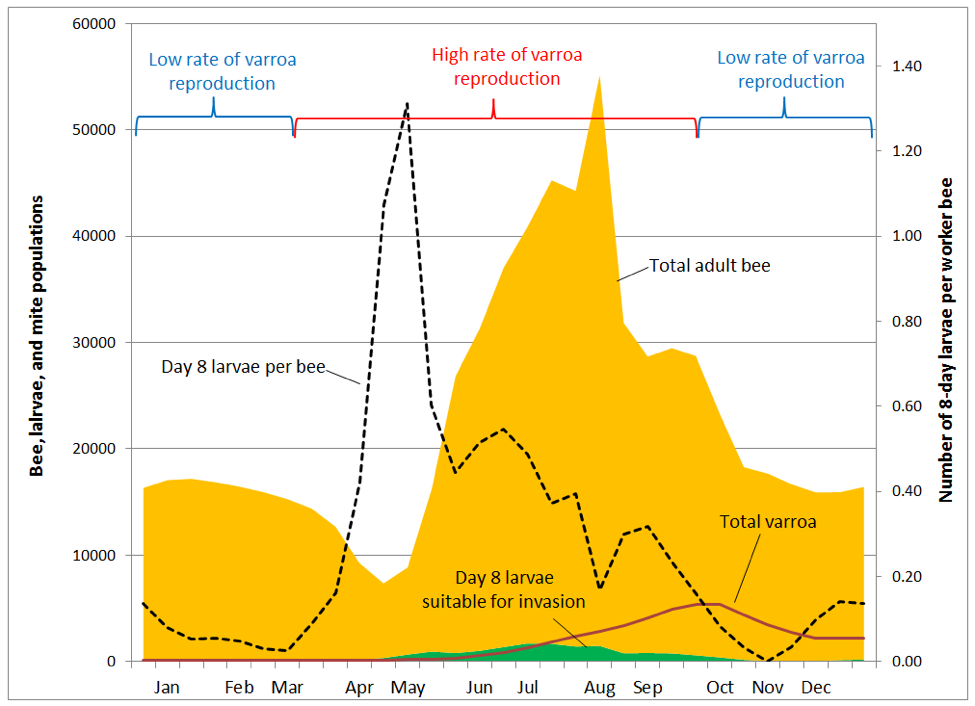
Practical application: you get the most bang for your buck by controlling varroa early in the season and during peak broodrearing. Once the main flow is over, varroa has already gained so much momentum that treatment is far less effective.
Update Jan 2017: I have developed a new mite model, which fine tunes the graphs used in this series of articles. I hope to publish it in early 2017.
Understanding Mite Monitoring
The “momentum” of varroa mentioned above is problematic, since it occurs invisibly, and doesn’t become apparent until it may be too late. Even the gold standard of mite monitoring (the alcohol wash) can give a false sense of the true level of mite infestation of the hive (by it appearing lower than it actually is).
In order to illustrate the situation, I tweaked the chart above to show how varroa is distributed in the hive over the course of the season (Fig. 8).
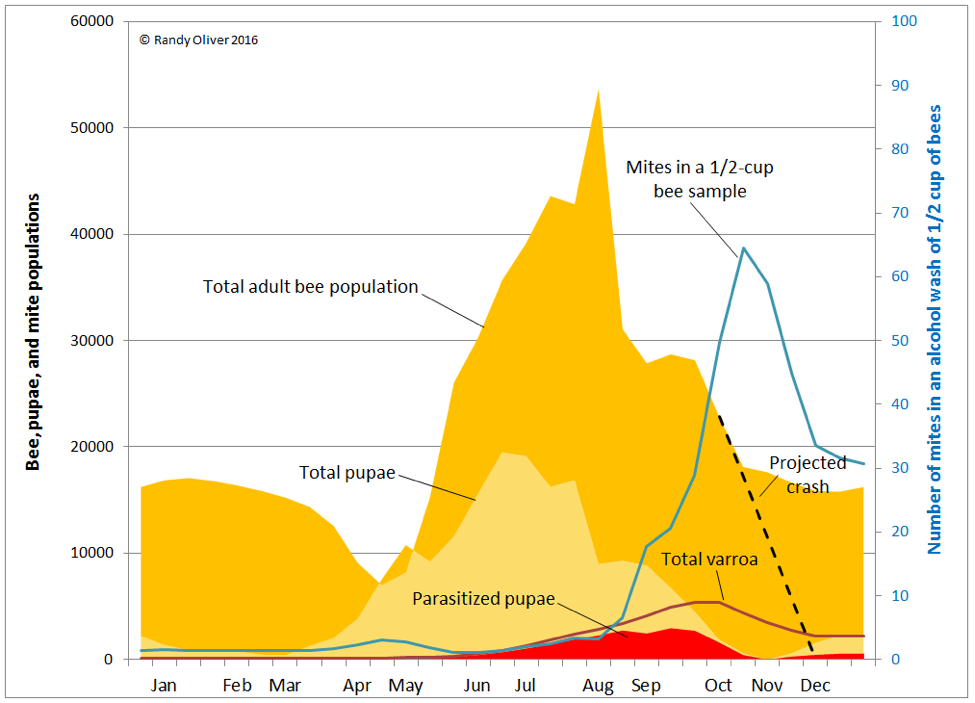 In the above graph, note how varroa are essentially a non-issue until the colony begins rearing drone brood in late May (not shown). By early August a quarter of worker pupae are parasitized, rising to 50% in mid September, and 85% in October. Tragically, the diligent beekeeper may not even notice that varroa is gaining the upper hand, since an alcohol wash of a half cup of bees in early August would give a count of only 3 mites (a 1% infestation rate). Alcohol wash counts wouldn’t start to climb noticeably until the mid-August count of 7 jumped to 18 two weeks later, and then rocketed skyward thereafter.
In the above graph, note how varroa are essentially a non-issue until the colony begins rearing drone brood in late May (not shown). By early August a quarter of worker pupae are parasitized, rising to 50% in mid September, and 85% in October. Tragically, the diligent beekeeper may not even notice that varroa is gaining the upper hand, since an alcohol wash of a half cup of bees in early August would give a count of only 3 mites (a 1% infestation rate). Alcohol wash counts wouldn’t start to climb noticeably until the mid-August count of 7 jumped to 18 two weeks later, and then rocketed skyward thereafter.
Practical application: the problem is that mite counts from adult bee samples do not reflect the number of mites hidden in the brood, nor the proportion of brood now infected by viruses. Despite the fact that the total number of mites in the hive less than doubles from mid August to the October peak, alcohol wash counts over that time period go quickly from only 3 up to 65 mites. This would give the erroneous impression that the mite population had exploded during that time.
In reality, the mite population had been steadily growing, unnoticed, and all hell is about to break loose. The damage has already been done, and even if the beekeeper manages to blast the mites into oblivion, the virus epidemic may remain self perpetuating—taking months for the colony to recover, if at all.
If this occurs during a pollen dearth, or following exposure to ag pesticides or miticide-contaminated combs, or with a poor queen, or with nosema, the problem is exacerbated, since the colony may not be able to recruit its way out of trouble by rearing a new round of uninfected bees.
Next
I’ll continue with what occurs next as viruses run rampant in the hive, as well as discussing “mite bombs, neglectful beekeepers, and the problem with mite immigration from other hives.
Aknowledgements
I thank my collaborator Peter Borst for always being at the ready to help me in my literature research. And to Lloyd Harris for the use of his data. And a hats off to those pioneering varroa researchers whose invaluable work may help us to eventually come to terms with varroa.
Notes and Citations
[1] In the southern latitudes of the U.S., where winters are mild and plants are blooming, colonies may grow even during winter. In those areas, there may be no broodless period, and the chart in Fig. 2 may need to have the winter (blue through purple colored areas) clipped out, and the remaining portions stretched to fit the local environment.
[2] This chart was created from data collected by Lloyd Harris, as described earlier in this series.
[3] Schmehl, DR, et al (2014) Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). Journal of Insect Physiology 71: 177–190. The researchers found that bees fed mixed pollens were less susceptible to chlorpyrifos toxicity.
[4] Smart, MD (2015) The influence of mid-continent agricultural land use on the health and survival of commercially managed honey bee (Apis mellifera L.) colonies. http://conservancy.umn.edu/handle/11299/171752
Also: Kirk Anderson has recently completed a study of colonies in the Dakotas (in prep) that came to a similar conclusion.
[5] Reviewed in: Sponsler, DB and RM Johnson (2015) Honey bee success predicted by landscape composition in Ohio, USA. PeerJ 3:e838; DOI 10.7717/peerj.838
[6] There’s an excellent video of a presentation on this subject by Dr. Amy Toth at https://www.youtube.com/watch?v=q-AUK4y34Ik
[7] Not to mention with civilization and environmentalism.
[8] Nazzi, F and Y Le Conte (2016) Ecology of Varroa destructor, the major ectoparasite of the Western Honey Bee, Apis mellifera. Annu. Rev. Entomol. 61:417–32. Yves Le Conte is one of the world’s most important long-time researchers on varroa, who coupled with one of the best and brightest next generation researchers to write this review. Unfortunately, the paper is behind a paywall.
[9] Bee and mite curves were plotted at 15-day intervals, with independent r values for each interval, based upon measured values.
[10] Of the phoretic mites—equivalent to a strong treatment with Taktic.
[11] A sampling of papers by Boot, Beetsma, and Calis:
Boot WJ, et al (1994) Factors affecting invasion of Varroa jacobsoni (Acari: Varroidae) into honeybee, Apis mellifera (Hymenoptera: Apidae), brood cells, Bull. Entomol. Res. 84: 3-10. I highly recommend this paper. Unfortunately behind a paywall.
Boot WJ, et al (1995) Why do Varroa mites invade worker brood cells of the honey bee despite lower reproductive success?, Behav. Ecol. Sociobiol. 36: 283-289. This paper is of special interest to anyone interested in breeding for mite resistance.
Boot WJ, et al (1992) Differential periods of Varroa mite invasion into worker and drone cells of honey bees, Exp. Appl. Acarol. 16: 295-301.
Boot WJ, et al (1993) Invasion of Varroa jacobsoni into honey bee brood cells: a matter of chance or choice?, J. Apic. Res. 32: 167-174.
Boot WJ, et al (1994) Behaviour of Varroa mites invading honey bee brood cells, Exp. Appl. Acarol. 18: 371-379.
Boot WJ, et al (1995) Does the time spent on adult bees affect reproductive success of Varroa mites?, Entomol. Exp. Appl. 75: 1-7.
Boot WJ, et al (1995) Further observations on the correlation between attractiveness of honey bee brood cells to Varroa jacobsoni and the distance from larva to cell rim, Entomol. Exp. Appl. 76: 223-232.
Boot WJ, et al (1995) Invasion of Varroa jacobsoni into drone brood cells of the honey bee, Apis mellifera, Apidologie 26: 109-118.
[12] 8-days from the laying of its egg for a worker larva; 8-9 days for drone larvae. Mites prefer, if given the opportunity, drone brood.
[13] Free, J B (1960) The distribution of bees in a honey-bee (Apis mellifera L) colony. Proceedings of the Royal Entomological Society of London (A) 35: 141-141.
van der Steen, JJM, et al (2012) How honey bees of successive age classes are distributed over a one storey, ten frames hive. Journal of Apicultural Research 51(2): 174-178.
[14] I divided the previous 12-day period’s sealed brood count by 20 to arrive at the number of Day 8 larvae susceptible to invasion by a mite.
[15] I used varroa intrinsic rates of reproduction (r values) from the literature, applying them to reflect the mite buildup rate in today’s typical commercial stocks. I guesstimated the percentage of mites in the brood ranging from 80% when the brood:worker ratio was high (easy for mites to find suitable cells to invade), down to 0% when there was no brood. Percentages of parasitized pupae and mites in an alcohol wash could then be calculated by simple math.