Reevaluating Beebread: Part 2 – The Players
Reevaluating Beebread: Part 2
The Players
Randy Oliver
ScientificBeekeeping.com
First published in ABJ Nov 2015
CONTENTS
Why Do Bees Go To Such Effort To Prepare Pollen In This Manner?
Which Microorganisms Are Involved?
Establishment Of Nest And Gut Microbiota
What About Antibiotics?
Next
Acknowledgements
References And Notes
In the last two decades, the widespread application of genetic and genomic approaches has revealed a bacterial world astonishing in its ubiquity and diversity [1]. It’s become clear that the superorganism that we call the honey bee colony includes a number of symbiotic bacteria. Some appear to be involved in nutrition and immunocompetence, but we are only beginning to understand which players are involved, and what they do.
In the last installment, I introduced Dr. Kirk Anderson. When hired by the Tucson ARS lab (on the downside of the CCD epidemic) he really did his homework, and boned up on everything known about the honey bee microbiome, from which he summarized the then current state of knowledge thoroughly and succinctly in an open-access paper [2], from which I’ll lift a few snips:
The hive of the honey bee may be best characterized as an extended organism that not only houses developing young and nutrient rich food stores, but also serves as a niche for symbiotic microbial communities that aid in nutrition and defend against pathogens. The niche requirements and maintenance of beneficial honey bee symbionts are largely unknown, as are the ways in which such communities contribute to honey bee nutrition, immunity, and overall health.
Kirk pointed out that ants, termites, aphids, and other insects cultivate beneficial bacterial and fungal symbionts, but that:
Given the depth of understanding in the aforementioned systems, it is alarming to consider that virtually nothing is known about the beneficial microbial symbionts of the honey bee, a social insect vital to the worlds food supply. Many thousands of different microbial strains have been cultured from honey bee colonies [he credits Dr. Martha Gilliam], yet we know virtually nothing of honey bee microbial ecology.
Kirk then laid out a vision of a lab devoted to furthering our understanding of how the naturally occurring microbiota of the honey bee colony contribute to bee health and nutrition. Let me be clear that the Anderson Lab is not the only group looking at the bee microbiome—in the U.S. alone, research in this nascent field is also being performed in the labs of Drs. Nancy Moran, Irene Newton, Jay Evans, Gene Robinson, and Quinn McFrederick [3], among others. This is spawning a new generation of bee researchers with proficiency in state-of-the-art technique and analysis.
It’s abundantly clear that we have a great deal yet to learn about the importance (or lack thereof) of the microbiota associated with the honey bee. But for now, let’s return to the subject of this article: the role of the microbes involved in the process of fermenting raw pollen into beebread. Such fermentation could be for either (or both) of two reasons—for simple preservation, or to enhance its nutritional quality. As Kirk explains [4]:
Nutrient conversion is readily distinguished from food preservation (storage). Although both processes have associated microbial communities, the primary function of food preservation is to prevent microbial degradation… preservation environments composed of plant material (e.g. silage) are typically dominated by Lactobacillus spp. and other acid tolerant microbes. Conversely, nutrient conversion involves an extended time component following the collection of plant matter, massive nutrient turnover prior to consumption and a vertically inherited mutualistic microbe or small community of microbes that emerges as the dominant force in the conversion of recalcitrant plant material.
The questions that arise are then:
- Why do bees go to such effort to prepare pollen in this manner?
- Which microorganisms are involved, and do the bees inoculate the beebread with specific symbiotic microbes?
- What chemical and biological processes occur during the fermentation process?
- What are the nurse bees’ consumption preferences?
- Is the fermentation necessary to release the nutrients of the pollen?
- Is the pollen made more nutritious during the fermentation process?
In a relatively short period of intense investigation, Kirk and his associates were able to obtain (at least preliminary) answers to most of the above questions, published in an open access paper [5]. So let’s cover each of the above questions in turn.
Why Do Bees Go To Such Effort To Prepare Pollen In This Manner?
Let’s first consider the dynamics of pollen within the hive. Pollen is the predominant source of protein and other colony nutrition; a continual supply is thus critical if brood is to be reared. Unfortunately, that supply is completely dependent upon an ever changing bloom and capricious weather. When colonies are in their rapid growth phase, or during pollen dearths, there is often little pollen to be seen in the combs. But that doesn’t mean that the colony doesn’t have a reserve stored. So where is it?
In the previous installment I described how the pollen foragers place their loads into cells adjacent to the broodnest, after which mid-aged bees pack it into place for storage. But this then creates the problem of how to protect such a rich larder of stored food (at the warm temperature of the broodnest) from the slew of pollen-feeding insects, mites, fungi, and bacteria eager to consume or decompose it. So what to do?
As Kirk observes, “nutrition stored within the body of an individual is protected by anatomical barriers and active immune physiology.” So nurse and mid-aged bees ravenously consume that freshly-stored pollen, thus protecting it within their bodies (Fig. 1).
Figure 1. Forager bees (sampled from the entrance) tend to have very little pollen in their guts, but “house bees” (nurses and mid-aged bees) tell a different story. I took this sample of 50 bees from an outside frame of the upper hive body in early November (after 2 days of confinement by rain), froze them to kill them, and then crushed them with a roller to squash out their gut contents. Note that the gut of every single bee was full of pollen. In other samples taken during the summer pollen dearth, a small proportion of bees from similar combs lacked pollen in their guts.
A question: The pollen in the above photo was mostly in the hindguts of the bees (post normal digestion). I wonder why they retain the remnants of digested pollen grains for so long, rather than defecating immediately. Could it be that additional nutrients are released or generated by the bacteria that thrive in the rectum?
Nurse and mid-age bees digest that consumed pollen and glandularly convert it into jelly (analogous to mammal milk)—which can be considered as the in-hive “currency” of protein, to be equally shared according to need. Any excess is converted to vitellogenin and stored in the fat bodies of all but the foragers. The colony is thus able to distribute protein reserves throughout the colony population as a whole.
In good times though, foragers bring in more pollen than the nurses can consume, so the colony must preserve it in some additional manner. They do so by fermenting it into beebread, using a process similar to that used for the creation of silage or sauerkraut—by allowing lactic acid bacteria to produce their preservative namesake, thus creating an environment hostile to other microorganisms, allowing for the relatively long term storage of pollen for later consumption.
The question then, is exactly…
Which Microorganisms Are Involved?
First, current day genomic research into the hive microbiome is not something that you do with a microscope. The literature is incredibly jargon-heavy, tedious to read, and consists of monster spreadsheets of numbers meaningless to all but those well versed in this arcane field (I’m definitely not one of them). But there is some common terminology and methodology that I need to briefly explain.
Until recently, scientists’ ability to study the microorganisms in the bee gut was largely limited to culturing them in petri dishes, ala Martha Gilliam’s meticulous research. The problem with that methodology is that many gut bacteria are difficult to culture. It was only with the development of PCR (polymerase chain reaction) to amplify scraps of DNA that we could start to identify organisms by parts of their genome alone. This technology was quickly followed by “metagenomics,” which allowed the identification of unknown and unculturable microorganisms from a sample of biological material. By convention, researchers compare differences in the “spelling” of the 16S ribosomal DNA genes present [6] in order to estimate how many different “species” of bacteria are present. From this analysis, they can then create phylogenetic “trees” of relationships. To their amazement, scientists found that we had missed about 99% of species of bacteria in various environments.
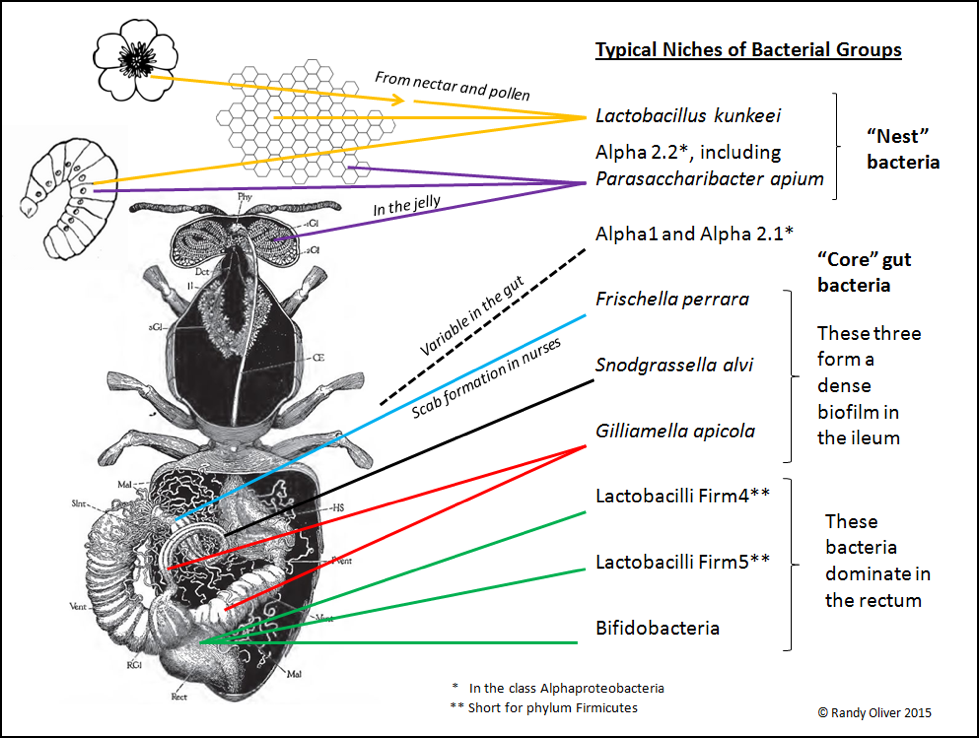
Only recently (2003 [7]) did researchers note that there appeared to be a distinctive gut microbiome in the honey bee, furthered by Diana Cox-Foster’s metagenomic analysis of colonies suffering from CCD [8]. We now know that honey bees tend to harbor a fairly consistent community of symbiotic bacteria [9,10], consisting of what appears to be around eight different “phylotypes” (the separation of bacteria into species is not as clear cut as it is for higher organisms [11]; bacteria identified only by DNA analysis are separated into OTUs (Operational Taxonomic Units, each having a group name such as “Firm 5”). In any phylotype, there may be several clades of OTUs (each OTU presumably being a closely-related species or strain, likely adapted to a slightly different niche, with a different biochemical profile, and thus possibly involved in a different symbiotic relationship, or performing different functions) [12]. As researchers refine their methods, they will surely further split these OTUs into additional named species. Each type of bacteria typically occupies a specific niche in the bee (or hive). Based largely upon an excellent recent review by Dr. Nancy Moran [13] and communication with Kirk Anderson, I created the graphic below to show where they are typically found (Fig. 2).
Figure 2. An adult honey bee may harbor a billion bacteria, with roughly 95% residing in the waste material in the hindgut; few survive in the microbe-hostile crop. Foragers bring environmental bacteria from flowers back to the hive–notably Lactobacillus kunkeei, which then thrives in the fructose-rich nest environment (especially in beebread). Other bacteria are nest symbionts, such as the Alpha 2.2 group found in jelly. And then there are the “core” gut bacteria, exhibiting strict niche fidelity within the bee gut.
Establishment Of Nest And Gut Microbiota
Let me first make clear that the research community is at a very preliminary stage at understanding the establishment and functions of the honey bee microbial community. Bacteria and fungi (as well as pathogens [14]) are brought into the hive with the nectar and pollen. Due to colony hygiene and the unfavorable environments of jelly, beebread, and honey, many species do not survive for long. But some find niches to their liking. And then there are other symbiotic bacteria which may be unique to the hive environment or bee gut—apparently transferred from one generation of bees to the next. And although there are “core” and “typical” communities of bacteria in the bees and the hive, they may vary substantially from colony to colony, race to race, and location to location [15].
Of interest is that Kirk recently found that the newly-emerged bees do not need to be exposed to older bees in order to acquire their inoculum of core bacteria—they apparently ingest it from the combs during their first few hours after emergence [16].
Kirk’s associate Lana Vojvodic tracked the establishment of bacteria in the larval gut [17], and Kirk recently found [18] that the worker gut microbial community structure is quickly established in young bees, with the core bacteria showing up within hours. Then over the next few days a process of succession occurs, with pioneer species establishing first, followed by a climax community that persists for the rest of the bee’s life (despite the huge change in diet as bees progress from nursing to foraging). Another group of researchers [19] recently found that the queen bee microbial community follows a similar course of development–with a huge difference–the queens, which are fed only jelly, develop a different climax community than that of the workers who feed them, consisting predominately (and not surprisingly) of the Alpha 2.1 and Alpha 2.2 bacteria associated with jelly.
The Alpha 1 and 2 phylotypes are as yet poorly understood. Researchers have now figured out how to culture some of these bacteria in the lab, so we may soon learn more about them. It’s likely that the bee and the bacteria have coevolved mutually-beneficial symbioses, presumably with natural selection tweaking the bee hindgut epithelial cells to favor colonization by favored bacteria, which may help with the digestion of honey and pollen, produce important molecules not already present in the food, modulate immunity to pathogens, or aid in the metabolism of plant (or agricultural) toxins.
Biology is always a dynamic work in progress, and there is continual evolutionary adjustment of any system. This appears to be occurring (on the evolutionary time scale) with bees and their symbionts. Symbiotic relationships typically begin as parasitic invasion or opportunistic exploitation of a food source (as in undigested gut contents), which may then evolve into commensalism, and eventually mutualism (see box).
|
Types of Symbiotic Relationships A parasitic relationship is one in which one member of the association benefits while the other is harmed. Commensalism describes a relationship between two living organisms where one benefits and the other is not significantly harmed or helped. Mutualism or interspecies reciprocal altruism is a relationship between individuals of different species where both individuals benefit. In the case of bees and bacteria, the bees provide a habitat and food source, and in turn the bacteria likely aid in digestion, immunity to pathogens, creation of essential nutrients, and detoxification of plant chemicals or pesticides. Definitions from Wikipedia |
In the case of the evolution of the honey bee microbiome, certain bacteria invaded the bee gut and fought to establish a niche. Of course, the bee would try to flush out harmful invaders, but some figured out how to adhere to the gut cells and establish a biofilm [20]. The bee and each species of bacteria then evolutionarily worked out some sort of agreement, presumably in which both species benefitted (mutualism).
The least refined relationships appear to be with the Alpha 2.2 Acetobacteraceae and Lactobacillus kunkeei, which are facultative symbionts—meaning that they can exist in other niches (such as in plant nectar), but some strains also thrive when bees create a hospitable habitat either within their guts or elsewhere in the hive (as in the jelly or beebread). Then there is Frischella perrara, an early invader of the guts of young bees, which causes formation of a scab (see the great photomicrographs at [21]). This bacterium may be transitioning from a parasitic to perhaps a commensal relationship. Finally, there are the highly refined and apparently mutualistic relationships with Snodgrassella and Gilliamella (and of the two species with one another). These species are perhaps only found only in the bee gut, where they form biofilms in the nutrient-rich ileum (where the bee absorbs the digestion products of pollen from the midgut). We do not yet know to what extent the bees depend upon them for health or survival.
One might ask why there are so few groups of bacteria in bees compared to the thousands associated with humans. One researcher suggests that it may be because mammals have a more developed adaptive immune system than do insects (by creating antibodies), which allows for the regulation of more complex relationships [22], or perhaps the abundance of real estate in the mammalian gut simply provides a greater number of distinct neighborhoods for bacterial colonization.
We are only beginning to grasp the complexity of the system; this is where Kirk’s lab is focusing its attention—to try to understand where bacteria and fungi fit into the hive environment as a whole. What I really appreciate about Kirk is that he’s scientifically skeptical (as am I) of nearly everything claimed about honey bees until he’s seen it tested and confirmed. So his lab has gone back to the beginning, assuming nothing, and confirming or refuting several previous “findings” regarding the hive microbiome.
For example, Kirk’s associate Vanessa Corby-Harris continued Vojvodic’s research, and recently named a species of Alpha 2.2 bacteria (Parasaccharibacter apium) which she found in the jelly produced by the hypopharyngeal glands of nurse bees [23]. Of interest is that jelly is clearly strongly antibacterial, yet she found bacteria that not only thrive in jelly, but confer mutualistic benefit to the larvae. Her research suggests that the source of inoculum of symbiotic bacteria to larvae may be the jelly, rather than the contents of the crop.
Practical application (probiotics): to date, there is only suggestive research that the gut symbionts confer appreciable benefit to the bee (this will surely become a hot topic of research). Nevertheless, there are plenty of sellers eager to market “probiotics” to beekeepers. The two predominant bacterial phylotypes in the bee gut are unnamed Lactobacilli, in the same group of bacteria that ferment yogurt, sauerkraut, and pickles. But don’t think that feeding yogurt, pickles, or any off-the-shelf probiotic will help your bees, since each of the many types of Lactobacillus is very specific to a certain niche. There will likely eventually be proven products on the market, but I don’t know of any as of yet.
What About Antibiotics?
Similar as to in your gut, the community of bee gut microbiota are in a constant competitive battle for real estate. If you enjoy street tacos in Mexico as I do, you likely have experienced what happens when a new player enters the neighborhood in your gut. Similarly, the application of antibiotics to your gut (or a hive) can shift the balance by (temporarily) knocking out some susceptible species. As suggested by Dr. Nancy Moran:
It would seem prudent to avoid overuse of antibiotics, as this could have detrimental consequences for colony health, just as chronic use of antibiotics might affect human health by continually perturbing resident gut communities.
But the gut bacteria don’t give up easily, and eventually incorporate genes for resistance to regularly-used antibiotics. This has happened with the U.S. managed bee population, following decades of exposure to oxytetracycline [24].
Does this hurt the bees? Perhaps at first, but I remember that back before varroa, when many of us treated twice a year with oxytet, we sure had healthy hives. A recent experiment by Drs. Eric Mussen and Brian Johnson didn’t find any negative effect on the growth of colonies after feeding a blast of antibiotics [25].
Practical application (antibiotics): there are two extreme views—(1) bees should never be fed antibiotics, and (2) antibiotics should be given prophylactically twice a year to prevent disease. Those promoting View 1 have apparently never had a sick kid whose life was saved by a course of antibiotics; the others are likely wasting money on risk management, chancing contaminating their honey with residues, and breeding antibiotic-resistant bacteria (keep in mind that only when some beekeepers created constant exposure to the antibiotic by using extender patties did we finally see AFB bacteria develop resistance to the treatment).
I suspect that much of the money spent on fumagillin, oxytet, and tylosin is not cost effective, and would better be spent on better nutrition and mite management. On the other hand, when actual monitoring indicates that the presence of a pathogen exceeds a threshold value, treatment can be of great benefit. This was the case in my operation in January, when EFB showed signs of going rampant. We treated all our hives prophylactically for the first time in over a decade, and were pleased with the results. But I have no intention of doing so next season unless clearly called for. The reasons? It’s expensive and time consuming to treat, and I want the antibiotic to still be effective the next time I need it.
Next
In the next installment we’ll delve into the details of what occurs during the fermentation of pollen into beebread.
Acknowledgements
I thank Pete Borst for his generous assistance in literature search, and Dr. Kirk Anderson for the great deal of time spent in discussion and explanation of this research.
References And Notes
[1] McFall-Ngaia, M, et al (2013) Animals in a bacterial world, a new imperative for the life sciences. PNAS 110(9): 3229–3236. http://web.stanford.edu/~fukamit/mcfall-ngai-et-al-2013.pdf This excellent review covers our current state of knowledge of symbiotic bacteria in general (not just for bees).
[2] Anderson, KE, et al (2011) An emerging paradigm of colony health: microbial balance of the honey bee and hive (Apis mellifera). Insect. Soc. 58: 431–444. http://naldc.nal.usda.gov/download/57606/PDF. This is the best open access paper to read for a deeper understanding of the subject, including details of honey bee digestion, state-of-the-art genetic sequencing technology (and discrepancies in analysis), descriptions of the eight “core” bacterial groups, and the microbial niches present in the hive environment. Note that this is a very hot topic these days, with new research coming out monthly.
[3] These researchers communicate informally, as well as by reviewing each others’ work, as explained in a recent blog by Quinn McFredrick: https://melittology.wordpress.com/2012/04/03/bee-microbiome-initiative/
[4] Snipped and paraphrased from Anderson, KE, et al (2014) Hive-stored pollen of honey bees: many lines of evidence are consistent with pollen preservation, not nutrient conversion. Molecular Ecology 23: 5904–5917.
[5] Anderson, KE, et al (2014) ibid.
[6] For you techies: for phylogenetic classification, the 16S ribosomal RNA gene is commonly used because of its ubiquitous presence in all living organisms, its small size, and its alternating structure of highly conserved (slowly evolving) and hypervariable regions. Researchers can analyze either the DNA or the RNA, which can then suggest the functions of each species (metabolism of certain sugars, creation of vitamins, digestion of cellulose, detoxification of plant chemicals, etc.).
[7] Jeyaprakash A, et al (2003) Bacterial diversity in worker adults of Apis mellifera capensis and Apis mellifera scutellata (Insecta: Hymenoptera) assessed using 16S rRNA sequences. J Invertebr Pathol 84:96-103.
[8] Cox-Foster (2007) cited in previous installment of this series.
[9] Martinson VG, et al. (2011) A simple and distinctive microbiota associated with honey bees and bumble bees. Mol Ecol 20:619–628.
[10] Moran NA, et al (2012) Distinctive gut microbiota of honey bees assessed using deep sampling from individual worker bees. PLoS ONE 7(4): e36393. doi:10.1371/journal.pone.0036393
[11] Newton, I (2012) The utility of bacterial nomenclature. http://microdiv.blogspot.com/2012/08/the-utility-of-bacterial-nomenclature.html
[12] Engel, PE, et al (2012) Functional diversity within the simple gut microbiota of the honey bee. PNAS 109(27): 11002–11007.
[13] The best current review of this is by Nancy Moran (2015) Genomics of the honey bee microbiome. Current Opinion in Insect Science 10: 22–28.
[14] Graystock, P, et al (2015) Parasites in bloom: flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc. R. Soc. B 282 http://dx.doi.org/10.1098/rspb.2015.1371 This exemplary experiment demonstrated how quickly and easily pathogens are transmitted between colonies via flowers.
[15] Disayathanoowat, T, et al (2012) T-RFLP analysis of bacterial communities in the midguts of Apis mellifera and Apis cerana honey bees in Thailand. Journal of Apicultural Research 51(4): 312-319.
Vojvodic S, SM Rehan, KE Anderson (2013) Microbial gut diversity of Africanized and European honey bee larval instars. PLoS ONE 8(8): e72106.
[16] The timing suggests that the inoculum may come from the meconium ingested during cell cleaning (which would imply that those bacteria remain viable over the 12 days post pupation), or from the nectar or honey consumed for the bee’s first meal, since newly-emerged workers typically do not begin consuming beebread until their second day.
[17] Vojvodic (2013) Ibid.
[18] Anderson, K, et al (in press) Ecological succession in the honey bee gut: Colonization by core bacteria during early adult development.
[19] Tarpy, David, Heather Mattila, Irene Newton (2015) Development of the honey bee gut microbiome throughout the queen-rearing process. Appl Environ Microbiol 81:3182–3191.
[20] Stones, DH & A-M Krachler (2015) Fatal attraction: how bacterial adhesins affect host signaling and what we can learn from them. Int J Mol Sci 16(2): 2626–2640 (open access).
[21] Engel, P, et al (2015) The bacterium Frischella perrara causes scab formation in the gut of its honeybee host. MBio doi: 10.1128/mBio.00193-15
[22] McFall-Ngai M (2007) Adaptive immunity: Care for the community. Nature 445(7124):153.
[23] Corby-Harris, V, et al (2014) Origin and effect of Alpha 2.2 Acetobacteraceae in honey bee larvae and description of Parasaccharibacter apium gen. nov., sp. nov. Appl. Environ. Microbiol. 80(24):7460.
[24] Tian, B, et al (Moran lab) (2012) Long-term exposure to antibiotics has caused accumulation of resistance determinants in the gut microbiota of honeybees. http://mbio.asm.org/content/3/6/e00377-12.full
[25] Johnson, BR, W Synka, WC Jasper & E Mussen (2014) Effects of high fructose corn syrup and probiotics on growth rates of newly founded honey bee colonies. Journal of Apicultural Research Volume 53, Issue 1: 165-170.