The Case of Pristine® And The Dying Queen Cells – A Pesticide Mystery Solved?
THE CASE OF PRISTINE® AND THE DYING QUEEN CELLS– A PESTICIDE MYSTERY SOLVED?
First published in ABJ March 2013
Randy Oliver
ScientificBeekeeping.com
A combination of cooperation between beekeepers and a pesticide company, good sleuthing, good science, and good luck may have solved the mystery of the dying queen cells. Let me walk you through the investigation.
The Case of Pristine Fungicide
If you hang out in the Northern California almond orchards during bloom, you’re going to see fungicides being sprayed over the trees, the bees, and the hives (and sometimes the beekeepers!). Due to our wet winters, growers spray hundreds of tons of fungicides during bloom (Fig. 1).
Figure 1. The spraying of fungicides is a common sight in almond orchards during bloom. Beekeepers beg their growers to spray in late afternoon or at night, when there is little pollen left in the blossoms, but during narrow treatment windows between rains, the sprayers (including helicopters) run nonstop all day long.
These fungicides don’t normally kill adult bees to any great extent (although the adjuvants in the tank mix may), but they (or again the adjuvants), may have adverse effects upon the brood, as reported by Dr. Eric Mussen in 2008 [1]:
California beekeepers seem to observe more problems with fungicide toxicity to their bees than beekeepers around the rest of the country. Perhaps that is because California beekeepers devote significant time to “lifting lids” in spring (actually, late winter by the calendar). As early as the late 1950’s beekeepers noted brood loss, in some apiaries, following the use of captan. Later, they noted brood loss following the use of Rovral®. Now, they report seeing brood loss following Pristine® applications. These are not immediate losses, such as one might see with Monitor® or other insecticides that are toxic to bee brood. These losses are noted, usually, about seventeen days after exposure. Counting backwards, that means exposure of one-day-old larvae that interfered with immature development. Pupae and newly emerged bees are seen with anatomical malformations, like undeveloped wings.
However, most almond pollinators take such occasional setbacks due to fungicides in stride, since colonies build up vigorously on almonds and generally recover quickly from the loss of a few foragers or a bit of brood. In my experience in almonds, I’d say that colonies that go in looking great generally come out looking fantastic! And I don’t notice any lingering effects due to exposure to the fungicides. But Mussen continues:
Does this mean that beekeepers can dismiss concerns over certain fungicides? No, not all beekeepers. If beekeeper observations are correct, Pristine-contaminated pollen, if consumed by colonies in the process of rearing queens, will decrease the number of queens reared to adults.
Problems in Queen Country
The California queen breeders, generally located in almond country, produce a large proportion of the nation’s queens each spring. They typically rear them in cell builders fed with combs of recently-stored almond pollen. In recent years, some of them have experienced occasional mysterious failures of entire batches of queen cells. Such failures could set a producer’s entire delivery schedule back for weeks during the critical early spring market for queens (this is no reflection on California queens—the vast majority of cell builders are unaffected).
The affected breeders noticed that such failures appeared to be linked to combs of pollen that had come from orchards that had been sprayed with the recently-introduced fungicide Pristine. Beekeepers were already leery of fungicides, after Eric Mussen’s warnings, especially since the Tucson Lab confirmed that some fungicides were toxic to bees [2].
Subsequent research by Dr. Gloria DeGrandi-Hoffman ‘s team suggested that Pristine might amplify the negative effects of the organophosphate insecticide chlorpyrifos upon bee nutrition, endosymbionts, and brood survival, including that of queen cells [3],[4],[5]. It appeared to be a slam-dunk case against Pristine!
So the queen producers put pressure on the growers not to use Pristine, which the growers then reported to their pest control advisers, who told the salesmen for the product. This of course got the attention of the manufacturer, BASF, who commendably sent out representatives to work with the queen breeders.
What appeared to be an airtight case against Pristine actually had some serious holes. For one, BASF showed us data from their research which indicated that Pristine, at field realistic doses, did not kill brood. Furthermore, plenty of healthy cells were being produced from pollen that did contain residues of Pristine. So we had opposing evidence as to whether or not Pristine was actually the problem.
Judging The Case
This is where it is useful to apply Koch’s Postulates [6]. The first question was whether Pristine was always associated with the unusual queen failures. Unfortunately, those failures were sporadic, and samples of beebread had generally not been taken, which made it harder to link residues of the active ingredient to the problem. And it was really hard to tell which hive had been exposed to what, since there are often several almond growers within flight range of any hive, and queen producers bring in combs from the field to stock their cell builders. So much for direct linking evidence.
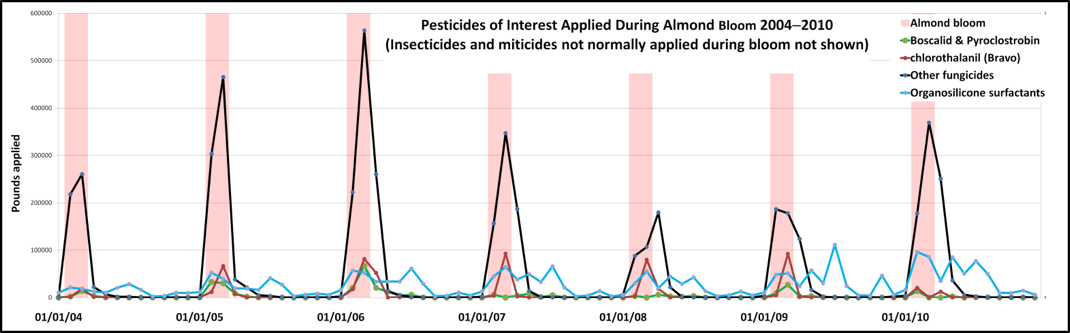
This is where California Pesticide Use Reports might shed some light. California has the best pesticide use reporting in the nation, with every commercial application recorded by applicator, date, crop, and location. These reports are summarized in an open data base, with more detail available from the local ag commissioners (who, in Northern California are very supportive of the beekeeping industry). I’ve graphed out the monthly application rates of pesticides on almonds below (Fig. 2).
Figure 2. Plot of applications of the major pesticides during almond bloom periods (in pink) for 2004-2010 (I actually plotted back to 2000 for reference, but the graph was too large for the page). The horizontal lines indicate each100,000 lbs of fungicide applied.. I apologize for the small detail, but you can see that the amount of other fungicides (black) dwarfs that of Pristine (green), which was first applied in 2004. I also broke out the fungicide Bravo (maroon) and the organosilicone surfactants (blue), both of which saw increased application after 2004. Thanks to Dr. Reed Johnson for supplying me the original database from California DPR.
The queen producers started complaining about lost cells around 2008. Note that little Pristine was applied statewide that year—far less than in 2005 and 2006. However, the breeders are not necessarily affected by statewide use, but rather only by applications immediately adjacent to their hives. I have not yet gotten that data for Pristine, but some of the queen producers are also almond growers, so they generally knew what pesticides were being applied (or so they thought).
Last season, BASF stepped up their efforts, and sent scientist Christof Schneider over to California to work with the queen producers and to take samples of pollen and beebread. Sure enough, residues of Pristine could often be found in pollen, but not in the royal jelly, suggesting that there would be little actual exposure to queen larvae.
BASF then went a step further, and attempted to fulfill Koch’s third postulate to see if they could experimentally create queen cell failure by intentionally feeding Pristine to cell builder colonies. They spent a lot of money last summer to run a “semi field” trial in which they grew flowering phacelia in hoop houses, sprayed it during bloom with Pristine, and then set up cell builder colonies inside the tunnels. Again, it was easy to detect residues of the active ingredients in the beebread, but not in the royal jelly. Most notably, the cell builders had no trouble producing healthy queens. This finding forced us to question whether the case against Pristine was as solid as we had assumed. So what else could be causing the queen cell failures?
A New Clue!
At this year’s California Beekeepers convention, I had the chance to review results from pesticide analyses of beebread samples from colonies that the queen producers had deployed in almond orchards during bloom last year. A few items caught my attention:
- In the first place, the California queen breeders have relatively pesticide-free comb. The breeders go out of their way to minimize pesticide and miticide residues, to the extent that they hold hives back from pollination to get “clean” combs of beebread.
- Surprisingly though, the beebread often still showed residues of coumaphos and fluvalinate, despite the fact that the producers hadn’t used those miticides in years!
- There were also residues of various other pesticides.
- But the real red flag was the unexpected presence of an “insect growth regulator”! This surprising discovery suggested a new suspect. An insect growth regulator (IGR), as its name implies, could well be harmless to adult bees, but might prevent queen pupae from being able to molt into adults!
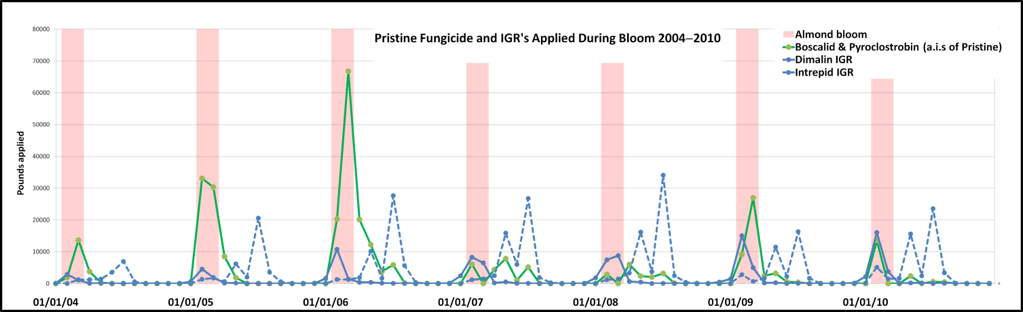
The IGR was diflubenzuron, generally sold by the trade name “Dimilin.” The queen breeders were surprised to hear that an IGR was being applied during bloom. Upon further checking I found that Dimilin had been approved for use in almonds and stone fruits since the 2004 season (Fig. 3).
Figure 3. Applications of Pristine (green) and two insect growth regulators (Dimilin (solid blue) and Intrepid (dotted)), both of which were first used in 2004. There was indeed a spike in Dimilin application in 2009 and 2010 (unfortunately CalDPR has not yet released 2011 or 2012 data). For simplification, I am referring to the active ingredients by their trade names, although in this data set I can’t be sure if other formulations were used.
Well-meaning pest control advisers were recommending Dimilin to control Peach Twig Borer, and the timing of application in the above graph suggests that growers may have been adding it as a tank mix with fungicides:
Growers have long relied or dormant oil sprays alone or mixed with organophosphate or pyrethroids for early-season Peach Twig Borer control. However, organophosphates are coming under increasing scrutiny of regulators who want them phased out. These sprays also have caused problems by disrupting beneficials and washing into waterways… “As a farmer I am concerned about what I use. I look for products that are not only effective, but safe on beneficials and do not harm bees.” [7]
The grower was correct in that Dimilin is safe for adult bees, but it certainly isn’t for brood! In a 2001 review, Tasei [8] concluded that:
Insect growth regulators, used for pest control management will cause no damage to adult honey bees and probably other adult pollinators, and can be considered as safer for foragers than second generation insecticides.
However, he also noted that:
This safety for pollinators is only apparent, since serious damage to brood has been reported in honey bees and bumble bees…Abnormal mortality in eggs, larvae or pupae and typical malformations have been observed in colonies after some IGR applications. These troubles were due to the properties of these products, which all interfere with embryo development and moulting process and which contaminate food resources collected (nectar and pollen) and stored in the colony by foragers. As a consequence, the effects of intoxication by IGRs are always delayed.
Additional recent research [9] has confirmed that Dimilin can cause brood mortality in honey bees and bumblebees. So here we are—the growers are trying to use a more environmentally-friendly product, but due to circumstance and unfortunate timing, they might be hurting a specialized but important segment of the beekeeping industry.
Getting Down to Details
So how much Dimilin is actually in the beebread? One queen breeder had several beebread samples analyzed in 2009, so I asked him for copies. There was only one sample with residues of Dimilin, but it was present at nearly 2000 ppb (Fig. 4)—a concentration at which larval mortality might realistically be expected.
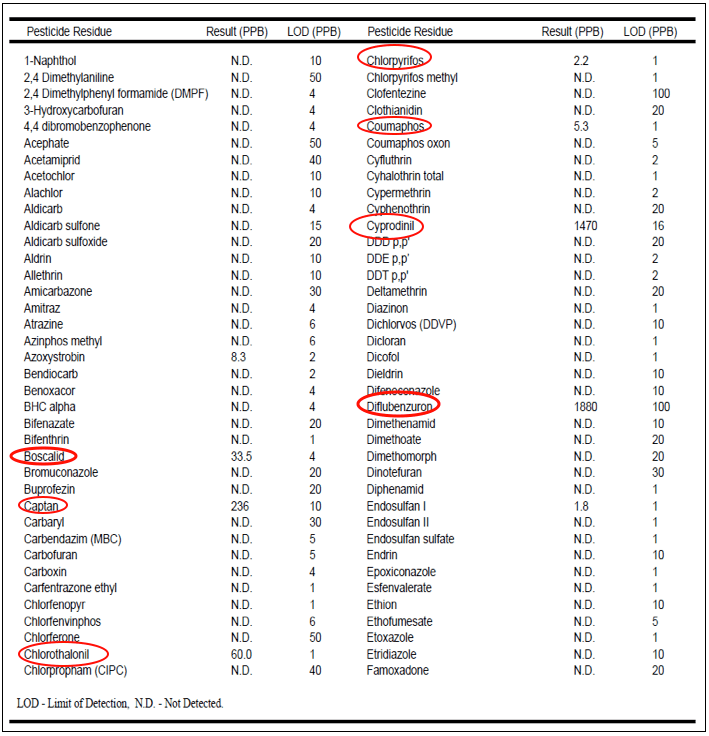
Figure 4. First page of a pesticide analysis of the beebread from a starter/finisher frame, with chemicals of interest circled. Note the detect for one of the active ingredients of Pristine—Boscalid—at a low level. But also note the residues of the fungicides captan, chlorothalanil, and cyprodinil (and on the second page, the fungicide iprodione came in at 6000 ppb). But the stunner is the presence of two insect growth regulators–diflubenzuron (Dimilin) at 1880 ppb, and methoxyfenozide (Intrepid) at 80 ppb (not shown)! Encouragingly, most samples showed very low levels of pesticides overall.
Clearly, Dimilin had been applied at least once next to at least one of this queen breeder’s hives in 2009, and gotten into the beebread.
Could Dimilin Be the Culprit?
The symptoms that the queen producers reported were poor “take” of grafts, or that the queens would develop through the pupal stage, and then die as they were in the process of changing into adults. When the producers uncapped the cells of the dead queens, they would find white or colored pupae, or deformed adults. Pay attention, because this is where it gets really interesting!
Dimilin is a “chitin synthesis inhibitor,” meaning that it arrests the formation of an insect’s exoskeleton, a process that is critical as a pupa metamorphoses into an adult. This is what makes it more environmentally safe—it only kills developing insects, not the adults, or other forms of life (although it is extremely toxic to aquatic invertebrates).
Koch’s First Postulate — Was Dimilin Associated With Queen Failures?
Breeder Ray Olivarez Jr. was curious as to how much Dimilin had been applied during bloom in the heart of queen country–Glenn County–so he paid for a detailed pesticide use report for Dimilin, from which I created the following graph (Fig. 5).
Figure 5. Gallons of Dimilin applied by month; almond bloom period indicated in pink. The use reports indicate that applications of Dimilin were scattered irregularly throughout the county (number of applications shown above bars). Have we nailed the culprit for the sporadic queen cell deaths? Note that when I checked the use report for another IGR—Intrepid—1000 pounds were applied to almonds in 80 different applications in the county in 2010.
So Dimilin was indeed applied during the years of queen failures—especially in 2009! Now jump ahead to 2012–of the 22 beebread samples taken last spring, 8 had residues of Dimilin, at maximum levels of 4000 ppb and average levels of 1600 ppb! These sporadic detections may explain why only a few breeders were seeing problems. The case against Dimilin is firming up!
Koch’s Third Postulate — Can Dimilin Experimentally Cause the Observed Symptoms?
But to clinch the case we need to see whether exposure to Dimilin will produce the same symptoms in queen cells that the producers observed. Thanks to my buddy Peter Borst, we were able to get our hands on a copy of an obscure article from a German beekeeping journal [10]. In Germany Dimilin was used for a number of years to control gypsy moths in forests, with no reports of bee mortality. But in the early 1990’s a queen producer starting noticing unusual failure of queen cells after a neighboring fruit grower applied Dimilin. The beekeeper then collaborated with university researchers to test the effects of Dimilin upon the development of queen cells.
Three trials were carried out in free-flying cell builders in March and June, testing Dimilin at 4 concentrations, each applied in a droplet of water added to the jelly surrounding 3-day-old larvae. The addition of Dimilin clearly caused queen cell mortality, and followed a dose response curve. The photographs of the dead developing queens were strikingly similar to those reported by the California queen producers!
Unfortunately, due to the mode of application in the experiments, we can’t directly compare the concentrations used with those found in beebread in California. One curious observation, though, was that queen cell survivability was much better in the mid-June trial, suggesting that there may be a seasonal component to Dimilin toxicity to developing queens. The successfully-emerged queens were placed into nucs, and appeared to function normally. Note also that the larvae were only fed a single dose of Dimilin, which then had a greatly delayed affect upon their survival.
Now for an interesting twist. Dr. Reed Johnson decided to perform his first study as a newly-minted PhD on the effect of Pristine (not Dimilin—we’ve gone back to Pristine) on queen cells (to see whether he would independently replicate BASF’s results indicating that it was indeed safe).
He got funding from Project Apis m, and collaborated with Sue Cobey and the Koehnens to set up several “swarm box” cell builder colonies (no free flying bees) so that he could completely control their diet. He spiked pollen with Pristine for the test hives, and fed plain pollen to the negative control group (for details, see [11]). And then as a positive control, he spiked pollen with an insecticide known to be toxic to brood. By stroke of luck, he chose to use an IGR–Dimilin (not yet knowing that Dimilin residues were later to be found in the samples taken from the queen breeders)! (If you’re getting lost, we’ve now got a researcher testing both the fungicide Pristine and the insect growth regulator Dimilin side by side).
Johnson presented his results far from the almond orchards–at the American Bee Research Conference in Hershey, PA. He confirmed the results of BASF’s tunnel trial that the fungicides in Pristine degrade rapidly in beebread, do not make it into the royal jelly, do not cause queen cell mortality, and do not affect the size of the resulting queens. Looks like Pristine is likely off the hook as far as the queen breeders are concerned!
As expected, the beebread spiked with the IGR Dimilin caused major queen larval and pupal mortality. But he had spiked at 100 ppm, which is equivalent to 100,000 ppb—far above the “field realistic” level. So Johnson plans to repeat the experiment this season at more realistic doses to see whether he can duplicate the exact symptoms of the failed queens as experienced by the breeders.
Other Factors to Consider
I spoke at length with Ray Olivarez Jr., who pointed out that some beekeepers in Texas also reported problems with rearing queen cells after returning from almonds. Ray notices that if there is a good pollen flow following almond bloom, that colonies may hold stored almond pollen in reserve—not digging into it until much later in the season (at times of dearth, when colonies may be nutritionally stressed). The implication is that pesticide residues in almond pollen could conceivably have greatly delayed effects upon brood or queen production (swarming or supersedure cells) much later in the season!
An even scarier finding came to light when I searched the literature for effects of IGR’s upon emerged queens and drones. Thompson [12] found that these insecticides may cause reduced egg survivability, drone loss, and reduced egglaying by the queens. The authors also suggested that IGR’s had the potential of causing long-term effects:
However, even if only those bees reared within 2 weeks of the IGR being applied are subject to premature ageing, this might significantly reduce the size of over-wintering colonies, and increase the chance of the bee population dwindling and dying in late winter or early spring.
Lest I raise unwarranted alarm, I checked the database of pesticide residue detections in pollen by Mullin [13]—the infrequent detections and low levels of residues of IGR’s suggest that beekeepers in general have better things to worry about. That is not to say that IGR’s couldn’t be a problem in certain areas!
Honey Bees Aren’t the Only Bees!
Beekeepers and almond growers may forget that there are also other species of bees involved in almond pollination. A recent study out of UC Davis [14], and worth reading] found that almond nut set was substantially better if other species of bees were present besides honey bees. The other bees apparently cause the hired honey bees to cross rows of trees and thus effect better cross pollination. Due to their life cycles, solitary- and bumblebees are much more likely to be susceptible to IGRs. Should the application of IGR’s prevent the native bees from successfully rearing reproductives, those species could quickly disappear from the orchard lands, to the detriment of the almond growers.
Where Does the Investigation Stand Now?
The results of two recent experiments, one by the registrant, and one independent, both appear to exonerate Pristine as a direct suspect for queen cell mortality. However, we can’t ignore DeGrandi-Hoffman’s findings, especially since the symptoms of the dead queens that she observed were similar to those reported by the queen producers (dark, fully-formed adults dead in the cells). Her research certainly demonstrates the pesticides in the beebread can negatively affect the ability of a colony to rear queens. Of interest is that those effects appeared to be indirect—neither pesticide was detected in the royal jelly; the effects were perhaps due to the exposure of the nurses that fed the larvae their first meals of jelly.
However, due to her experimental design, it is difficult to interpret and directly apply her findings to the question of whether Pristine is directly responsible for queen cell losses:
- She used two pollen sources composed of entirely different species of pollen (mixed natural desert flora vs. pesticide-contaminated almond).
- She did not do a straight comparison of queen cell survival in which the presence of Pristine was the only variable.
- The pollen of both of her treatment groups were contaminated with a high level of the organophosphate chlorpyrifos—at nearly 1000 ppb in the pollen and 310 ppb in the beebread. Such levels were not typical of the many beebread samples that I’ve seen from the queen producers (in which chlorpyrifos was seldom detected, and if so, at very low levels).
- There is also the question as to whether the “dose” of Pristine that she used was “field realistic.”
And what were the actual field realistic residues of Pristine in Glenn County? In order to see, I looked at the five beebread samples analyzed in 2009—a year of high Pristine application (see Fig. 3). Four of the five contained boscalid (the “marker” active ingredient of Pristine)–at levels of 23, 33, 34, and 86 ppb. Of the 22 samples taken in 2012, the average level of boscalid was a considerably higher 264 ppb. But those levels were all much lower than the concentrations tested by DeGrandi-Hoffman (1835 ppb of boscalid in the pollen; 682 ppb in the beebread)).
So I’m not sure how to interpret her findings, other than concur with her conclusion that residues of Pristine may “amplify” the effects of chlorpyrifos.
In the two trials in which Pristine was tested alone, neither researcher used almond pollen, so any potential contributory effect of the almond phytotoxin amygdalin could not be accounted for (see my other article in this issue). In any case, BASF tested an even higher dose of Pristine than did Degrandi-Hoffman, but did not observe increased queen mortality.
And that level pales in comparison to the 100,000 ppb (in the pollen) used by Reed Johnson, at which he also observed no negative effects upon queens (again, without the presence of the additional organophosphate). These findings certainly suggest that Pristine alone is not to blame, and that the chlorpyrifos in DeGrandi-Hoffman’s pollen was more responsible for the cell mortality than was Pristine.
I’m sorry to be throwing so many numbers and chemical names at you, but this is the only way that we can find out which pesticides are actually causing problems, and which are benign. We beekeepers must continue to vigorously pursue the investigation until we nail the guilty party (or parties)! For that, we owe a debt of gratitude to the Tucson lab, Dr. Reed Johnson, BASF, and all the other parties involved.
So at this point we really can’t yet positively say what was to blame for the mysterious deaths of queen cells, although the case against Dimilin is certainly compelling. Reed Johnson may eventually demonstrate that it is indeed the IGR, or maybe not. The pesticide use reports suggest a few other potential suspects that have been applied in increased amounts in recent years, such as some of the fungicides. Or another IGR, Intrepid, which causes premature molting. Or perhaps the increased use of the organosilicone surfactants is allowing other pesticides to better penetrate the bees’ exoskeletons or guts. Or maybe there is some sort of synergy going on between the various pesticides. But we are on track to get to the root of this problem!
In the meantime, our Extension Apiculturist, Dr. Eric Mussen, wasted no time in getting the word out to the pest control advisers [15], most of whom seem to be willing to do what they can to keep from killing bees (not a bad idea, since the California almond growers spend some $200 million annually to rent live bees to pollinate their crop).
Although UC Davis pest management guidelines state that Dimilin can be applied as a bloom spray [16], they also state that it can be effectively applied prior to or after bloom. It would likely be in the best interests of almond growers to avoid spraying Dimilin during bloom for two reasons:
- Any factor that hurts the honey bee industry will be reflected in the prices that growers are forced to pay for pollination, and
- It appears that growers receive valuable service from native bees as well as honey bees, and should look to protect them as well!
Lessons Learned
Any beekeeper who runs bees to almonds and then raises queens afterward should be aware that in combs of stored almond pollen there may be pesticide residues that could affect queen cell development (as a personal note, for thirty years I’ve raised plenty of queen cells on almond pollen and have never noticed a problem).
Perhaps the most important thing to be learned from this story is that beekeepers shouldn’t be too quick to finger an “obvious” suspect. Based upon circumstantial evidence, we may have placed the blame on the wrong chemical! Although Pristine certainly appeared guilty, two studies have now confirmed that healthy queens can be reared on pollen containing Pristine at normal doses.
Practical application: The fact that we may have made a false accusation will not be lost upon the EPA, the pesticide companies, nor the growers–which should give us pause. We should take this as a sobering lesson for our industry to be careful about whom we blame for what!
As agriculture and the EPA move towards more environmentally-friendly pesticides, the Law of Unintended Consequences may come into play. It may be the case that in trying to replace the environmentally harmful oil/organophosphate dormant sprays in almonds, that the “reduced risk” IGR that are taking their place might cause the unintended consequence of affecting California’s queen producers. Note that even “natural and organic” pesticides have the potential to still cause problems for honey bees—for example, Thompson [17] notes that one of the components of Neem oil can apparently cause delayed serious winter mortality. Any change in pesticide applications, even if for the better environmentally, means that there will be a new learning curve involved!
It’s also important to note that the California beekeepers were able to collaborate with the pesticide manufacturer, who voluntarily spent a great deal of money to work cooperatively with us to solve a problem. BASF has expressed their willingness to continue to work with us (I find that other manufacturers are also more than willing to work with beekeepers). Beekeepers, through Project Apis m, funded an exemplary study by Dr. Johnson, showing that we can fund our own research– which could then be submitted to EPA to help with risk management decision making.
The case of the unexplained queen cell deaths is a real life whodunit. I hope that this article demonstrates the process, and the difficulties involved, in determining the actual cause of what appeared at first glance to be a simple pesticide issue. The story is not yet over. But we are learning a great deal as we try to solve the mystery!
Acknowledgements
Thanks to Peter Borst, Christof Schneider and Joe Wisk of BASF, Ray Olivarez Jr., Reed Johnson, and Eric Mussen for their help and comments.
References
[1] Mussen, E (2008) Fungicides Toxic to Bees? http://entomology.ucdavis.edu/files/147900.pdf
[2] Alarcón, R and G DeGrandi-Hoffman (2009) Fungicides can reduce, hinder pollination potential of honey bees. Western Farm Press March 7, 2009. http://westernfarmpress.com/fungicides-can-reduce-hinder-pollination-potential-honey-bees
[3] http://www.alfredstate.edu/files/downloads/New-York-Beekeepers-GDH.pdf
[4] http://www.wasba.org/newsletters/WSBA%20Jan%202012%20(revised).pdf
[5] DeGrandi-Hoffman, G, et al (2013) The effects of pesticides on queen rearing and virus titers in honey bees (Apis mellifera L.). Insects 4: 71-89. http://www.mdpi.com/2075-4450/4/1/71/pdf
[6] https://scientificbeekeeping.com/sick-bees-part-18b-colony-collapse-revisited/#kochs-postulates
[7] Cline, H (2003) State recently approved for use in tree crops: Dimilin OK’d for PTB control. http://westernfarmpress.com/state-recently-approved-use-tree-crops-dimilin-okd-ptb-control
[8] Jean-Noël Tasei, J-N (2001) Effects of insect growth regulators on honey bees and non-Apis bees. A review. Apidologie 32: 527-545. (free access)
[9] Thompson, HM, et al (2005) The effects of four insect growth-regulating (IGR) insecticides on honeybee (Apis mellifera L.) colony development, queen rearing and drone sperm production. Ecotoxicology 14(7):757-69.
Mommaerts, V, et al (2006) Hazards and uptake of chitin synthesis inhibitors in bumblebees Bombus terrestris. Pest Manag Sci.62(8):752-8.
[10] Nitsch C, et al (1994) Effect of Dimilin on queen rearing, Dtsch. Bienen. J. 2, 16–19 (in German).
[11] Johnson, R. & E. Percel (2013) The effects of the fungicide pristine on queen rearing. 2013 ABRC. Johnson fed pollen treated with four concentrations of Pristine (0.4, 4, 40 and 400 ppm), an organosilicone-containing spray adjuvant (Break-Thru, 200 ppm), the combination of Pristine and Break-Thru (400: 200 ppm), diflubenzuron (100 ppm) as a positive control or water as negative control.
[12] Thompson, HM (2005) Op. cit.
[13] Mullin CA, et al (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5(3): e9754. http://www.plosone.org/article/info:doi/10.1371/journal.pone.0009754
[14] Brittain C, et al (2013) Synergistic effects of non-Apis bees and honey bees for pollination services. Proc R Soc B 280: 20122767. http://dx.doi.org/10.1098/rspb.2012.2767
[16] http://www.ipm.ucdavis.edu/PMG/r3300211.html
[17] Thompson, HM (2005) Op. cit.