Sick Bees – Part 18F1: Colony Collapse Revisited – Pesticide Kills
Sick Bees Part 18F1
Colony Collapse Revisited
Pesticide Kills
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in January 2013
As long as I’ve been keeping bees, one of our worst fears has been that we might suffer a serious pesticide kill. Pesticides (especially insecticides) have always been, and will continue to be, a problem for bees and beekeepers.
Jim Doan of New York hadn’t experienced a serious pesticide kill in 25 years of keeping bees in corn/soy/alfalfa farmland. But when he approached one of his yards last spring, he smelled the stench of dead bees. What he saw made him sick to the stomach—piles of dead and rotting bees in front of every hive!
Jim related to me that he called his state’s Department of Conservation to investigate the kill, to no avail. So he contacted his State Apiarist, who sent out an inspector a couple of days later to take samples, which then languished in a refrigerator until at Jim’s request they were sent to Dr. Maryann Frazier, who in turn sent them to the USDA lab for analysis.
Although pesticide residues were found, no investigation was done. No applicator was reprimanded, and no fine imposed. And Jim’s losses weren’t covered by either his insurance policy or by ELAP [1].
Jim’s disaster was hardly an isolated case. I’ve spoken with a number of beekeepers who have suffered recent pesticide kills. Dave Shenefield’s bees were working white clover in Indiana at corn planting time. A farmer drilled treated corn seed directly into a field of flowering clover without first burning the weeds off with herbicide. The planting dust fell directly onto the blossoms being worked by the bees, poisoning his colonies as the foragers returned covered with toxic dust.
Darren Cox’s bees in Utah get hit regularly by applications of pyrethroids or carbamates onto flowering alfalfa hay. These applications done are despite label restrictions that clearly state:
“This product is highly toxic to bees exposed to direct treatment or residues on blooming crops or weeds. Do not apply this product or allow it to drift to blooming crops or weeds if bees are visiting the treatment area.”
Darren related to me a scenario: an aerial applicator, under a contract arranged perhaps two weeks earlier, loads up with insecticide, and flies 50 miles to treat the field. But when he gets there, he’s surprised to see that the alfalfa is purple with bloom. What’s he to do—turn around and unload, or just go ahead and spray anyway, knowing that such an action would be in violation of the label. But this is Utah, where the local primacy partner tends to turn its head to pesticide violations (Fig.1). You can guess the rest–such unnecessary and preventable bee kills frustrate Darren to no end!
Figure 1. A typical insecticide kill in Utah. Simple timely communication between the grower and the applicator as to the stage of bloom could prevent many such kills, as the applicator could then make more appropriate product application choices. Photo courtesy Jared Taylor.
I could go on and on, beekeeper after beekeeper. What I hear is that some states are better at others at enforcing pesticide regulations—it’s tough to be a beekeeper in those states that aren’t doing their job! To make things worse, beekeepers are often justifiably hesitant to pursue investigation, since in a number of states, complaining beekeepers have been fined for having illegal miticide residues in their hives! And if a beekeeper raises too much of a stink he could become persona non grata to the local landowners and lose his locations.
Farmers and applicators could often easily prevent bee kills by simply making sure that they spray before or after a crop comes into bloom, or by spraying after dusk with a product having a short residual toxicity, or by using a less bee-toxic product that is labeled for application during bloom. Such practices would eliminate a large proportion of bee kills, yet some farmers and applicators just don’t give a damn, and worst of all, get away with illegal applications (scofflaw applicators may consider any fines levied for pesticide misapplication as a minor business cost)!
What bothers beekeepers most is the unfairness of it. Ranchers (even of alpacas, reindeer, or emus) receive government benefits for livestock losses due to fire or severe weather [2], and beekeepers may be eligible for benefits for colony losses if they jump through the hoops of ELAP [3]. But neither of those programs cover losses due to pesticide application–either legal or in violation of the law.
Think about it–if someone poisoned a herd of cattle with pesticide overspray, it would make the news! You could damn well bet that the incident would be investigated and the applicator fined, and the cattleman would sue for damages via civil action. But this is generally not the case if your livestock are honey bees. Few damaged beekeepers receive any compensation at all for their losses.
Now I don’t want to give the impression that the pesticide situation is dire for all beekeepers. As I pointed out in a previous article [4], many beekeepers in agricultural areas have little or no problem with pesticides. And many commercial beekeepers simply shrug off the occasional bee kill as a cost of getting good locations in agricultural areas. However, in some areas of intensive agriculture, those commercial beekeepers who provide the bulk of pollination services tell me that pesticide issues are their major problem.
A Bit of History
In order to understand the run up to our current situation, it is helpful to read the engaging “Report on the Beekeeper Indemnity Payment Program” (which was in effect from 1967-1980) [5]. I’ll share a few excerpts:
During the mid-1940’s, [pesticide] damage subsided as farmers shifted from the use of arsenicals to DDT which is less toxic to bees. However, by the late 1960’s, use of DDT was decreased sharply because of insect tolerance to the poison. Finally, use of DDT and other chlorinated hydrocarbons was banned because of environmental concerns. In most cases, the highly toxic [organo] phosphates and carbamates were used in place of the banned sprays. This increased the problem of bee loss to the point of disaster for many beekeepers…
Partial colony losses are not always easy to detect…pesticides may weaken colonies to such a point that they do not survive the winter. This type of loss is often ascribed to winterkill rather than pesticides. Further, this loss may be extended to the replacement bees placed in contaminated equipment the next season. Often, not all losses are discovered soon enough after the chemical application to determine the exact cause of death.
Investigatory clue: these records of the field experiences of beekeepers prior to varroa are important to keep in mind, notably that there were “sublethal effects” from the pesticides that caused later winter mortality. I hear the exact same complaints from beekeepers in agricultural areas today. Clearly, varroa and beekeeper-applied miticides have added to the stress upon bee colonies, but elevated winter mortality due to pesticide exposure was the norm prior to the introduction of varroa.
Colony losses due to pesticides were severe in several states during the 1960’s. There was a “sharp decline in pesticide losses” in California during the early ‘70’s due to the state imposing “strict control of spray application”—only 54,000 colonies were killed in 1974, compared to 89,000 in 1970 (an improvement, but hardly cause for celebration). But then in the mid 1970’s, encapsulated insecticides (Penncap-M) were brought to market, again causing devastating losses when foragers dusted with the time-release particles returned to the hive and stored them in the beebread.
During June 1976, selected beekeepers in California and Washington were contacted to discuss the pesticide situation…Beekeepers in Washington report that there are no safe locations for bee yards. One beekeeper said, “No matter where I place my bees in the Yakima Valley, they will be sprayed at least once within ten days.” A beekeeper in the San Joaquin Valley of California described his efforts to protect his apiaries as “playing musical chairs with 40 loads of bees….” Several beekeepers said that even if they did move their colonies to another location, it could be sprayed the next day.
Practical application: I hear exactly the same words today from commercial operators. We have made great progress with pesticides since the 1960’s, but still not enough!
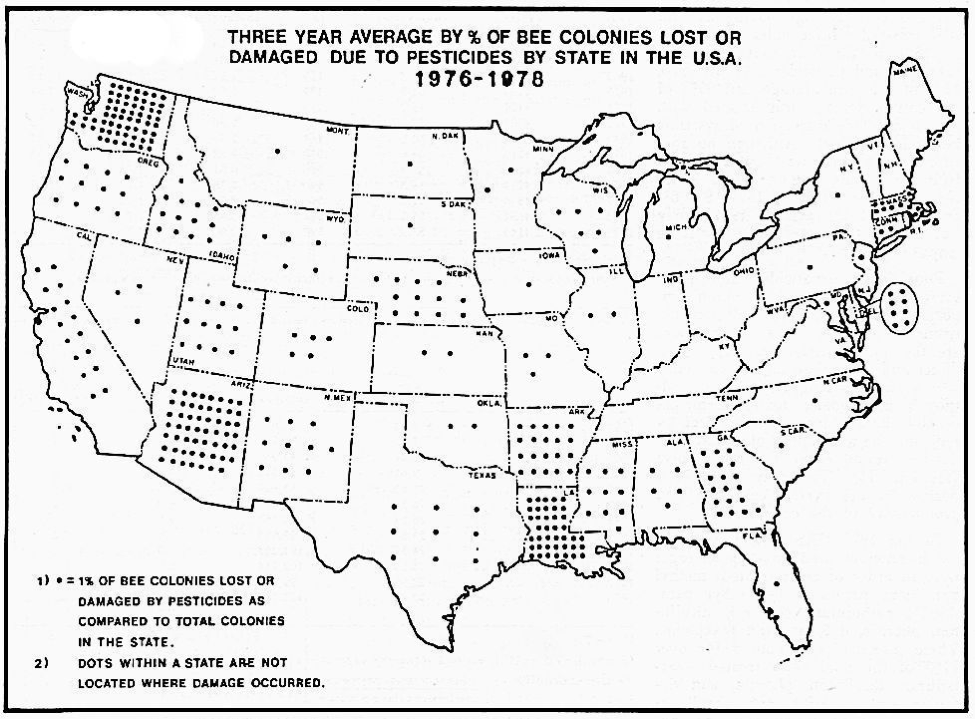
Beekeepers in Arizona, California, and Washington accounted for a large proportion of claims because they lacked access to “safe” forage areas (these were the early days of using forklifts in bee operations, and moving bees was hard work). It was not unusual for large beekeepers to suffer serious pesticide damage to half their hives each year, and they would likely have been unable to stay in business without governmental help (Fig. 2).
Figure 2. Back when the Agricultural Stabilization and Conservation Service kept records of reported bee kills for indemnification purposes (not all kills were reported), it was easy to see in which states pesticide applications were a serious problem. In recent years bee kills have not been tracked by any agency. Map from Erickson & Erickson 1983 [[i]].
[i] Erickson, EH, and BJ Erickson (1983) Honey bees and pesticides. ABJ 123(10): 724-730.
For nearly a decade, the Indemnity Program compensated beekeepers for pesticide losses. Those in only eight states filed the bulk of claims. As today, a small percentage of commercial beekeepers control the vast majority of colonies, and provide most pollination services. Well less than 1% of beekeepers in the country filed claims in any one year. By contrast, over 90% of the Arizona beekeepers in the program filed claims— not surprising due to the frequent spraying of the vast acreage of cotton suffering from a serious infestation by pink bollworm in the mid 1960’s [7], and the lack of alternative non-agricultural forage in that dry state.
The largest payment to a single beekeeper (name and state not specified) was $225,400 in 1972 (that would be $1 million in today’s dollars), and he filed for $228,000 two years later. You can imagine how this might not have set well with some budget-conscious congressmen!
And of course some crafty beekeepers learned to work the system:
On the other hand, some commercial beekeepers contend the indemnity payments have permitted, and in some cases encouraged, the survival of marginal beekeeping operations. The “marginal manager,” in this context, was characterized as any beekeeper who had become dependent upon indemnity payments as a source of income.
Those alleged “marginal beekeepers” reportedly left their hives in areas that they knew would be sprayed, and managed their colonies only enough to keep them barely alive so as to be able to collect more payments the next year (or kept collecting payments on deadouts). These fraudulent practices also did not play well to the program overseers.
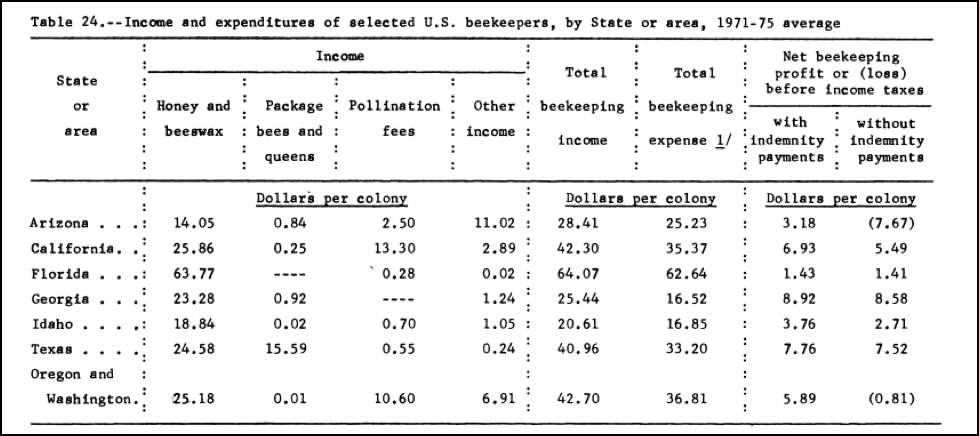
The study also looked at the profitability of beekeeping; I found one of the tables to be of particular interest (Fig. 3):
Figure 3. You can roughly adjust these figures into today’s dollars by multiplying them by five. What surprises me is that despite it being painfully costly to maintain colonies today in California (the annual expense being about $190) [[i]], the profit margin is substantially higher now than it was back then–not because of honey (since honey prices have only kept pace with inflation [[ii]]), but rather due to much higher pollination rates in almonds. Also of interest is that in those days beekeepers spent next to nothing on feeding syrup, and pollen supplement isn’t even mentioned!
[i] Mussen, E (2009) How much does it cost to keep commercial honey bee colonies going in California? http://projectapism.org/content/view/83/27/
[ii] http://www.nass.usda.gov/Statistics_by_State/California/Historical_Data/Bees.pdf
It is instructive that the analysts were aware of the cost to the beekeeper of pesticides:
This analysis shows that beekeeping income is affected most by severely damaged and destroyed colonies. Severely damaged colonies may require 6-8 weeks to recover colony strength. If the damage occurs during a major honey flow, the field force will be greatly reduced and honey yields could be lowered 60 percent or more. Severe damage in late summer may weaken a colony preparing for winter and increase the chances for significant winter kill…Beekeepers estimate it takes about one year for a destroyed colony to regain its income earning potential.
The authors conclude that without the indemnity payments, “farmers seeking pollination services would have to pay substantially higher rental fees to obtain bees.” Congress decided to pass that cost onto the farmers anyway, and terminated the indemnity program in 1980 (leaving some beekeepers with still-unpaid IOU’s). Today the almond growers bear the brunt of those higher rental fees; the huge number of colonies produced to meet the demand for high-paying almond pollination ensures that there are plenty of strong hives available for other crops afterwards.
Colonies generally come out of almonds in better shape then when they went in. This is not true for a number of other crops. The combination of poor forage and pesticides in several crops can weaken colonies to the extent that they don’t survive the season.
Allow me to close with some prescient conclusions from the report:
Unless Federal and State governments act ot regulate and caution applicators of toxic pesticides, colony damage will continue to be a major problem for beekeepers. However, most government officials emphasize that farmers and spray applicators are already confronted with enough regulations…the current development of stronger and longer-lasting pesticides…is creating an environment entirely unsuitable for honey bees in many parts of the U.S. These areas will find it harder to maintain the present level of bee population regardless of an Indemnity Program or higher honey and pollination prices.
Remember that the above words were written prior to the invasion of the tracheal mite, the varroa mite, Nosema ceranae, or the Small Hive Beetle—beekeeping hasn’t gotten any easier since their arrival!
Practical application: beekeeping in agricultural settings has always been a tough way to make a living. Fortunately, many beekeepers tell me that things have gotten better in their regions. But in some areas of intensive pesticide application, it’s hard to keep a hive alive from one year to the next.
Nailing Down the Guilty Party
This spring my bee operation suffered from a case of Sudden Forklift Collapse (Figure 4).
Figure 4. Early this spring I suffered from a case of Sudden Forklift Collapse. This was no “sublethal effect” and did not go unnoticed! In a forklift kill like this, it didn’t take Sherlock Holmes to determine that the cause of death was due to a falling oak tree. If only the causes of pesticide kills were so easy to pin down!
In my case of Sudden Forklift Collapse, the cause was evident. Such is often not the case with pesticide kills. You may not even see any dead bees if the field force is poisoned in the field and never makes it back to the hive. Perhaps (as in the case of planting dust) you only see a handful of young bees and drones dying at the landing board. Or maybe the brood turns spotty. If the pesticide disorients the foragers, you may wonder why you didn’t get the normal honey crop. Or maybe there is some sublethal effect from which the colony simply “slows down” for a few months, or doesn’t make it through the winter.
In any of those cases, it may be difficult, if not impossible, to nail down the culprit. You don’t know where your bees were foraging, and any pesticide application within a 3-mile radius is suspect. You may not immediately recognize that there was a pesticide problem at all, so any residues could be degraded or washed off by rain by the time you think to have the dead bees or beebread tested. And even if you happen to visit the yard immediately after the kill, good luck in getting an understaffed and untrained state or county agency to quickly come out and properly collect and freeze a good fresh sample. And even then the analytical tests cost so darned much!
Action item: aggrieved beekeepers often have VERY STRONG FEELINGS! However, in order to change pesticide regulations, the EPA needs incontrovertible evidence that a certain pesticide used according to label restrictions caused adverse effects to honey bees. We need any and all beekeepers who suffer from substantial pesticide kills to file an “incident report.” Such a report is most effective if it contains a photographic record, documentation that rules out other plausible causes for the dead bees (e.g., tracheal mites or starvation due to unusual weather or forage conditions), and chemical analysis of samples of bees and beebread, properly taken by a state agent. If your local primacy partner is unable or unwilling to help, you may report directly to http://www.npic.orst.edu/eco or beekill@epa.gov.
One would think that solving Jim Doan’s kill would have been straightforward, since there were fresh piles of dead bees in front of the hives. He hadn’t previously experienced serious kills in those yards, so something different had happened. There was no apparent change in plantings this year, but with commodity prices at an all-time high, a farmer might have felt that it was worthwhile to apply more or different insecticides as precautionary “risk management.” Surely it would be easy to find incriminatingly-high levels of the offending pesticide in the dead bees or combs.
According to Jim, due to unfamiliarity with the investigation of pesticide kills, the state inspector collected less than an optimal amount of bees for pesticide analysis. Two samples were later sent off to the USDA lab (the cost of analysis was split between Jim and Project Apism)—results below (Fig. 5).
Figure 5. Analysis report of the two samples from Jim Doan’s spring bee kill (column headings added).
Figure 5. Analysis report of the two samples from Jim Doan’s spring bee kill (column headings added).OK, so now Jim had a report. But what did it tell him? As for the dead bees, the 1.6 ppb* of clothianidin insecticide is far too low to have caused bee mortality (1.6 ppb = 0.16 ng/bee; the LD50 for clothianidin lies in the range of 22-44 ng/bee).
* To help with the math, LD50 = median lethal dose; 1 ppb = 1 part per billion = 1 μg/kg = 1 ng/g; μg = microgram (one millionth); ng = nanogram (one billionth); a bee weighs about a tenth of a gram, so for every 10 ppb of residues in a sample of dead bees, any bee on average would contain 1 ng/bee .
So how about the high dose of Captan fungicide? As best I can tell from the literature, “Studies on the honeybee using technical Captan fungicide indicate that the LD50 is greater than 10 μg a.i./bee, and that there is 9.8% mortality at 215 μg a.i./bee.” So let’s do the math: 1290 ppb = 129 ng/bee, or 0.129 μg/bee—so again, it would be hard to make a case that this chemical was responsible for the obvious pile of dead bees.
Maybe the analysis of the pollen sample from the comb might help. I have no idea as to how it was taken, which can make a huge difference (Fig. 6).
Figure 6. These are plugs of beebread that I pulled from a brood frame. Note the layering of the different species of pollen. If a colony suffers from a pesticide kill, any traces of the responsible pesticide residue may only be in the topmost layer of pollen. If the state agent who takes the beebread sample scoops all the way to the midrib, he may dilute the offending pesticide by a factor of 10 or more.
The one pollen sample from the one comb from one colony (get my point?) in Jim’s affected apiary contained 399 ppb of the organophosphate insecticide Phosmet. The contact LD50 for this compound is listed as 0.0001 mg per bee (= 0.1 μg/bee = 100 ng/bee). Surprisingly, there doesn’t appear to be any published oral LD50 for Phosmet to honey bees! By my math, the concentration of Phosmet in Jim’s pollen sample would not be expected to have killed his bees either, although since it is a violation of the label to spray the insecticide on flowering crops, one is left wondering how it appeared in the pollen.
So this is how it can be for a beekeeper and his innocent bees—the suddenly-appearing piles of rotting corpses in front of every one of his hives certainly suggest that his bees were killed by a pesticide application. Unfortunately, due to a lackluster investigation by the primacy partner, and lack of implicating chemical evidence, Jim will never know what or who was responsible for the kill, nor be compensated for his losses, if justified. And he has no idea whether the same thing will happen again next season!
To make matters worse, Jim’s bees apparently got hit again in July, resulting in piles of greasy-looking dead and twitching dying bees in front of the entrances. And as I write these words in November, Jim sent me yet another photo of hundreds of freshly-dead bees once again in front of the hives (despite him confirming low levels of varroa and nosema). Jim is now a justifiably frustrated and angry beekeeper–not only did he suffer considerable financial loss (not to mention the ugly death of his beloved bees), but no one learned anything from the experience! The unwitting farmer(s) have no idea whether their pesticide applications caused the problem, Jim’s state agencies aren’t making any particular effort to prevent the same thing from happening again next year, and EPA didn’t receive any useful adverse effects report. Yes, frustrating!
It is disturbing for me to present these facts. Our managed honey bees function as a conspicuous and charismatic indicator species for the effects of pesticides upon “non target organisms.” Yet some agricultural areas are a “no bees land” due to either inadequate label restrictions or flagrant violation of those restrictions. And keep in mind that the honey bee colony has the capacity to absorb pesticide kills that would exterminate solitary pollinators, such as native bees, butterflies, and beneficial insects.
Practical application: if honey bee colonies are being killed, we can safely assume that the situation is even worse for more sensitive species!
Keep ‘em Honest!
Let me share another quote from the Indemnity Report:
The Beekeeper Indemnity Program itself discourages civil court action…Greater use of the civil court system by beekeepers to seek compensation for pesticide losses could reduce applicator negligence.
There you have it! The sad truth is that it will take the push of lawsuits to ensure that our pesticide laws are actually enforced. Accordingly I’ve studied the judgments for some beekeeper lawsuits. Be forewarned that a successful lawsuit requires unimpeachable evidence and impeccable argumentation—so one should not enter into an expensive lawsuit lightly!
The AHPA has started a legal defense fund to pursue test cases against egregious violations of pesticide law, with the hope of setting legal precedent, as did Jeff Anderson’s successful lawsuit against the state of Minnesota in 2005 [10]. I’m hesitant to step into politics, but I feel that this is probably a good course of action that could help the cause of advancing pesticide regulation. We beekeepers must tread carefully here to avoid pissing off the farmers who allow us to place bees upon their land. In truth, I’d like to see Xerces or some other environmental groups filing such lawsuits, so that they, rather than beekeepers, would take the heat. However, action is preferable to inaction.
Action item: you may join me in contributing to the National Pollinator Defense Fund at http://pollinatordefense.org/site/?page_id=11
I wish that I could present a simple solution to this problem, but there isn’t one—especially since the U.S. is currently locked into the high-input large-scale monoculture agribusiness model. The good news is that EPA is on the side of the beekeepers and the environment [11], and that things are clearly getting better—the worst pesticides are being phased out, new “reduced risk” pesticides and “biological” are put on the EPA fast track in order to get them into the market, plus a new generation of “smart” robotic application systems are being developed. There has never been more public awareness of the plight of the honey bee, and beekeepers are awkwardly basking in the spotlight of being considered as environmental stewards. The bad news is that the process of reducing the damage by pesticides to non target species is hampered by, among other things, ignorance (and lack of enough good scientific data), politics, property rights, consumer demand, and Money (intentionally spelled with a capital M).
OK, that’s enough griping for now–let’s get back to an investigation into any connections between pesticides CCD.
Pesticides and CCD
Biological plausibility: pesticides can weaken the colony by killing or otherwise affecting the foragers, reducing adult bee longevity, having adverse effects upon the queen, brood, or nurse bees, or by affecting bee behavior. In addition, they could react with other toxins, beekeeper-applied miticides, or suppress the bee immune response to pathogens. Any of the aforementioned could conceivably result in colony dwindling, mortality, or collapse.
Residues in the Combs
Let’s narrow down our focus. CCD by definition is not the result of the sorts of acute pesticide kills detailed above. So what we are interested in is colony mortality or morbidity due to sublethal effects that hadn’t already killed bees outright! In the case of winter mortality, since few pesticides are applied at that time of year, and since colonies normally purge any remaining field bees during the “fall turnover” [12], we’d expect any contribution by pesticides to be from residues in the combs, where they should be detectable by analysis.
Making the Link
One would think that it would be a simple matter to make the connection between pesticide residues and winter mortality—simply analyze pollen and beeswax samples from the combs, and determine whether there is a correlation between residues of specific pesticides and colony mortality.
The above sounds so straightforward and easy, but in actuality this is where it gets complicated. My point of going into detail on the analysis of Jim Doan’s apparently obvious bee kill was that if it’s that hard to figure out exactly what caused an acute pesticide kill, imagine how difficult it would be to definitively link colony mortality to any sublethal effects from a specific pesticide!
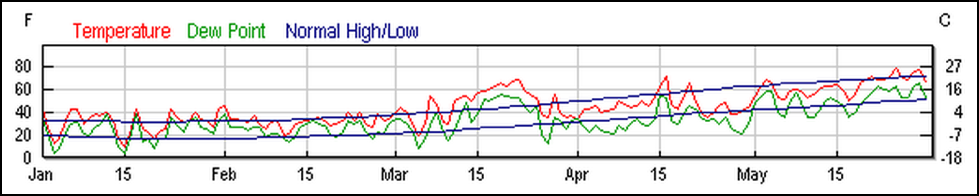
In fact, I took artistic license in greatly simplifying Jim’s story. In doing my usual fact checking, I found out that the actuality was complicated by personalities, politics, weather (Fig. 7), and a history of indemnity payments. To add further confusion, another beekeeper on the same farm did not observe any dead bees in front of his hives (but did notice that his nucs on that farm did not build up as well as those at other nearby locations).
Figure 7. Western New York experienced extraordinarily warm weather (followed by cold) in May. I find that such weather anomalies can result in piles of dead bees in front of hives due to short-term starvation events. Weather graph from www.weatherunderground.com.
However, I’m appreciative of Jim for sharing his observations and analysis report, and feel that it was a good example of the problems that researchers and regulators encounter as they try to figure out exactly how pesticides are affecting colony health.
These complicating factors may be why no scientific study has yet been able to firmly link colony mortality to pesticides. Here are the conclusions of all monitoring and analytical studies that I’ve seen to date:
- Germany: “As expected, the results show that pollen [from 210 hives sampled over 3 years] is contaminated with a plethora of chemical substances originating from the agricultural practice of using pesticides but also from the apicultural necessity of using acaricides… Accordingly, no relation between contamination of pollen and colony development or winter losses could be demonstrated in the course of the project although special emphasis was put into this aspect” [13].
- France: “Several cases of mortality of honey bee colonies (varying from 38 to 100%) were observed in France during the winter of 2005-6. In order to explain the causes of these mortalities, a case control study was conducted on a limited area, together with a larger survey in 18 other apiaries located in 13 sites over the entire country…No pesticide residues of agricultural origin were found in the samples of beebread, beeswax, honey and dead honey bees, with the exception of imidacloprid…found in one apiary [and] not considered to be able to cause honey bee acute mortality” [14].
- France: “A 3-yr field survey was carried out in France, from 2002 to 2005, to study honey bee … colony health in relation to pesticide residues found in the colonies… No statistical relationship was found between colony mortality and pesticide residues” [15].
- Italy: “The data obtained from the winter 2009-2010 inspections were used as the basis for chemical analyses on bee and wax samples, to test for residues of organophosphate, organochlorurate, carbamate and neonicotinoid pesticides, but no significant presence of these substances was detected” [16].
- Spain: “The present data [beebread samples from 12 apiaries] are in agreement with studies showing no negative effects of seed-treated crops. Some pesticide residues were found here, in particular several varroacides and insecticides, but no significant differences were observed between the different sunflower crop samples and those from the sites of wild vegetation. This fact not only implies environmental contamination but also supports the theory that, most of the time, inadequate [read that “unapproved”] treatments are the main source of residues that might weaken bee colonies and make them more sensitive to other factors” [17].
- Spain: “This study was set out to evaluate the pesticide residues in stored pollen from honey bee colonies and their possible impact on honey bee losses in Spain. In total, 1,021 professional apiaries were randomly selected… A direct relation between pesticide residues found in stored pollen samples and colony losses was not evident accordingly to the obtained results” [18].
- Europe (thorough review): “Currently there is no clear evidence from field based studies that exposure of colonies to pesticides results in increased susceptibility to disease or that there is a link between colony loss due to disease and pesticide residues in monitoring studies” [19].
- USA (CCD Descriptive Study): “This study found no evidence that the presence or amount of any individual pesticide occurred more frequently or abundantly in affected apiaries or colonies” [20].
- USA (2012 CCD Progress Report): “When pesticides are viewed in aggregate on a national scale, residues of pyrethroids …pose a threefold greater hazard to bee colonies than neonicotinoids, based on mean and frequency of detection in pollen samples and relative acute toxicity. The synthetic pyrethroid detected in the highest quantity and frequency in honey bee colonies that is used by beekeepers to control Varroa mite is tau fluvalinate” [21].
- USA (Stationary Hive Project) : “We did not find any relationship with any of our measures of pesticide contamination and colony loss rate at the apiary level for either 2009 or 2010” [22].
OK, I’m as puzzled as you are! It defies both common sense and a long history of beekeeper experience that researchers haven’t yet nailed down any link between pesticide residues in the combs and colony mortality! The above were not industry-funded studies, and several of the researchers started with a strong anti-pesticide bias (nearly all researchers suspect that pesticides are involved to some extent). And I’m certainly not about to tell you that pesticides/miticides and winter mortality are unrelated–it’s just, like I said, complicated.
I found that in order to begin to understand the effects of manmade pesticides upon bee health that I first needed to back up and examine some of the complex biology involved in natural bee/plant/toxin interactions. We’ll start in on that next month…
Acknowledgments
I’d like to thank the editor of this journal, Joe Graham, for giving me the latitude, support, and encouragement to write this series of articles. And a special thanks to Dianne Behnke of the publishing department for digging up and scanning archived issues of ABJ for my research.
References
[1] http://www.fsa.usda.gov/Internet/FSA_File/elap_honeybee_11.pdf
[2] http://www.fsa.usda.gov/Internet/FSA_File/lip2011_158c020211.pdf
[3] http://www.fsa.usda.gov/Internet/FSA_File/elap_honeybee_11.pdf
[4] https://scientificbeekeeping.com/the-extinction-of-the-honey-bee/
[5] ERS (1976) Report on the beekeeper indemnity payment program. http://babel.hathitrust.org/cgi/pt?id=coo.31924001799307;seq=8;view=1up
[6] Erickson, EH, and BJ Erickson (1983) Honey bees and pesticides. ABJ 123(10): 724-730.
[7] http://naldc.nal.usda.gov/download/48077/PDF
[8] Mussen, E (2009) How much does it cost to keep commercial honey bee colonies going in California? http://projectapism.org/content/view/83/27/
[9] http://www.nass.usda.gov/Statistics_by_State/California/Historical_Data/Bees.pdf
[10] Anderson v. State Department of Natural Resources Minnesotahttp://www.animallaw.info/cases/causmn693nw2d181.htm
[11] http://www.epa.gov/opp00001/ecosystem/pollinator/then-now.html
[12] Mattila HR, Otis GW (2007) Dwindling pollen resources trigger the transition to broodless populations of long lived honeybee each autumn. Ecol Entomol 32:496–505.
[13] Genersch E, et al (2010) The German bee monitoring project, a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41: 332-352.
[14] Chauzat MP, et al (2010) A case control study and a survey on mortalities of honey bee colonies (Apis mellifera) in France during the winter of 2005-6. Journal of Apicultural Research 49: 40-51.
[15] Chauzat MP, et al (2009) Influence of pesticide residues on honey bee (Hymenoptera, Apidae) colony health in France. Environmental Entomology 38: 514-523.
[16] Mutinelli, F, and C Porrini (2010) Report based on results obtained from the second year (2010) activity of the APENET project. http://ebookbrowse.com/apenet-2010-report-en-6-11-pdf-d189566755
[17] Bernal J, et al (2011) An exposure study to assess the potential impact of fipronil in treated sunflower seeds on honey bee colony losses in Spain. Pest Management Science 67: 1320-1331.
[18] Bernal J, et al (2010). overview of pesticide residues in stored pollen and their potential effect on bee colony (Apis mellifera) losses in Spain. Journal of Economic Entomology 103: 1964-1971.
[19] Thompson, HM (2012) Interaction between pesticides and other factors in effects on bees. http://www.efsa.europa.eu/en/supporting/doc/340e.pdf
[20] vanEngelsdorp D, et al. (2009) Colony Collapse Disorder: A Descriptive Study. PLoS ONE 4(8): e6481.
[21] http://www.ars.usda.gov/is/br/ccd/ccdprogressreport2012.pdf
[22] Drummond, F, et al (2012) The first two years of the stationary hive project: Abiotic site effects. http://www.extension.org/pages/63773/the-first-two-years-of-the-stationary-hive-project:-abiotic-site-effects