Neonicotinoids: Trying To Make Sense of the Science Part 1
Trying to Make Sense of It All
Looking at Both Sides of the Issue
Academic versus Field Applicable
Problems in Methodology and Interpretation
Neonicotinoids: Trying to Make Sense of the Science
Part 1
Randy Oliver
ScientificBeekeeping.com
First published in ABJ August 2012
Science is all about trying to understand things. When a scientist gets a hunch about why is it that something happens, he puts his hypothesis to the test in an experiment. He may then publish the results, including his own interpretation of the data—at which point other scientists are duty bound to question every aspect of the study, as well as to attempt to replicate the original results. In the end, we hope to learn what is actually true.
And this is my intent, to get to the truths of the neonicotinoid issue. In this series of articles, I am essentially “thinking out loud.” I find that the neonic issue is so emotionally charged that folk try to pigeonhole you as holding a black or white position, and then try to paint you as defending that position. Please let me be clear—I hold no position, and am not trying to defend anything! I’m simply asking that we stick to the facts, rather than playing to irrational fears and supposition. To that end I am intentionally taking on the role of “mythbuster,” which is predictably rubbing some folk the wrong way. But if I can get people actually thinking, rather than merely parroting, then I feel that my efforts have been successful!
So how do we reconcile the conflicting reports on the neonics? Last month I reported from Ground Zero of neonicotinoid use, and found the majority of beekeepers to be doing just fine. On the other hand, there was a rash of reports this spring of apiaries suffering serious mortality from planting dust. And to further confuse the issue, several recent scientific studies have been interpreted as having demonstrated that neonics are going to be the death of bees.
Trying to Make Sense of It All
As I reported in my last article, many beekeepers feel strongly that the widespread use of the neonicotinoid insecticides has been a good thing—there are far fewer spray kills nowadays than back in the bad old days (in 1968 an estimated 83,000 colonies were lost to pesticides in California alone [1]). However, there remain several unresolved issues and unanswered questions about these insecticides:
- There are occasional, but intolerable bee kills due to seed planting dust (especially so this year), from which individual beekeepers may suffer serious financial losses. This issue must be resolved!
- Although there is a substantial amount of good field data indicating that neonic residues in pollen and nectar are generally at tolerable levels, in some instances higher concentrations have been found. These situations need to be clearly identified.
- The long-term potential buildup of residues in soil must be carefully monitored.
- The sky-high application rates of neonics for landscape uses (turf, ornamentals, homeowner use) and on flowering trees, and the resulting runoff into surface waters, is of legitimate concern.
- The sublethal effects upon bee behaviors, such as memory, navigation, and age-related task allocation need to be further studied.
- The interactions between these insecticides and the bee immune response to parasites such as mites, nosema, and viruses need to be thoroughly investigated.
- Neonics have been shown to synergize with one particular class of fungicides. Other synergies should be explored, although there is no particular reason to suspect that neonics are unique in this matter.
All the above are things to be suspicious of, but to date there is no overwhelming evidence that any of them, save for the planting dust issue [2], generally cause serious problems. To date, no independent investigatory body has been able to confirm that the neonics are responsible for large-scale colony mortality [2].
Practical application: the scientific community and the regulatory bodies are well aware of the potential adverse effects of the neonicotinoids, are actively researching the issues above, and are in the process of reassessing their risks.
There is a growing public demand for more environmentally-friendly pesticides, which must be balanced against the real-world needs of agriculture for effective pest control products in order to feed a hungry world. Unfortunately, there are constraints due to cost and the expiration of patents that limit the actual amount of testing that can be done before regulators must make decisions as to whether a pesticide appears to be safe enough to be registered for use. Accordingly, the EPA often grants “conditional registration,” which allows it to ask for continued testing under actual field conditions. This is a good thing, since approved uses of a conditionally-registered pesticide can be quickly revoked should problems appear.
This is where independent scientists take over from those of the pesticide industry, and follow their hunches to test for any suspected negative effects that the pesticide might cause to “off target” organisms, such as humans and honey bees. The confusing part to the public is that…
Look and You Shall Find It
As with anything, the more you look, the more you will find potential risks (just Google the words “dangers of” followed by any food, medicine, or household chemical). You can drive yourself crazy with “what ifs.” The trick is to try to put all the findings into perspective.
Looking at Both Sides of the Issue
What I find, is that to be objective one must go out of one’s way to investigate all of the evidence, and to listen carefully to the interpretations by all parties. I already had a thorough grounding in distrust of pesticides, having come of age shortly after the publication of Rachel Carson’s seminal book, Silent Spring (which jumpstarted the environmental movement). I have a background in aquatic biology, and have clearly seen the devastating effects of pesticides and pollutants on downstream organisms. I’m deeply concerned about our overreliance upon pesticides and the resultant environmental consequences, well summarized by Dr. David Pimentel [3].
What I have also done, however, is to take a look at the issue through the eyes of the other stakeholders—the farmers and the companies that supply them with the plant protection products that they clamor for. I find that it often helps to play “Devil’s Advocate” and argue the “other side’s” position. I must admit that my doing so has gotten me into hot water with a number of beekeepers, but if our side can’t rebut the other side’s arguments, then we don’t really have a good case, do we?
What I found was that there are dedicated people already trying to objectively sort out the evidence. These are the regulatory agencies, such as the EPA, which are assigned the difficult responsibility of deciding how best to balance environmental safety with the demands of agriculture—a difficult task to say the least!
The Regulatory Gauntlet
Manufacturers screen each newly-developed chemical for any potential uses, including that as a pesticide. For a chemical (whether natural or synthetic) to be registered as a pesticide, the registrant must demonstrate both its efficacy against one or more pests, as well as its relative safety to both humans and to the environment as a whole. To do so it must run a gauntlet of tiered levels of “risk assessment.”
In general, a product is evaluated in a stepwise fashion, first (in the case of honey bees) to determine the degree of exposure (e.g., honey bees wouldn’t be expected to get into cockroach bait), and then to quantify the toxicity of the product by both contact (spray or contamination of leaves) and orally (as in nectar, pollen, or water). Risk assessment has been updated to take into account exposure to residues from systemic pesticides (such as the neonicotinoids), which are absorbed by plants and distributed in plant tissues, rather than simply sitting on the surface (Fig. 1).
Figure 1. Routes of exposure to systemic insecticides and potential effects upon honey bee colonies. Diagram © SETAC (2011) Pesticide Risk Assessment for Pollinators: Summary of a SETAC Pellston Workshop.
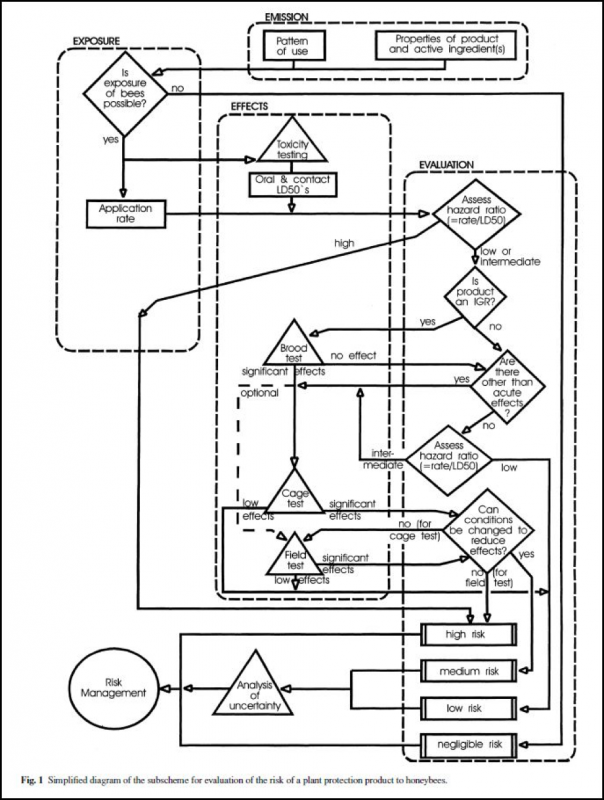
After determining whether there is a risk of bees being exposed to the product, the next tier of risk assessment is to determine the LD50 (median lethal dose) and the NOEL (no observed effects level) of the pesticide, for both oral and contact routes, and acute and chronic exposure, for adult bees as well as brood. Safety margins are then applied to decide whether the risks to either adult bees or brood indicate that additional testing is necessary to quantify sublethal effects. Testing is done first in the lab, then “semi field” (in enclosed screened tunnels over crop plants), and then under full field conditions (hives next to planted fields) (Fig. 2).
Figure 2. A simplified flow chart for the risk assessment of plant protection products. This diagram has since been updated to reflect the use of systemic insecticides (EPPO 2010, SETAC 2011). IGR means “insect growth regulator.” The EPPO, EPA, and SETAC documents are freely available on the web—I suggest that interested beekeepers read them! Chart © European and Mediterranean Plant Protection Organization (EPPO 2003), by permission.
Not all countries use exactly the same testing requirements, most notably that in the EU and Canada, the formulated product, as opposed to solely the active ingredient, must be tested (a position that I strongly support). I’ve sat with some of the principals and discussed the state of the art of testing. All parties (including Bayer) would like to improve the risk assessment protocols, and develop a standardized set which all countries (and manufacturers) alike could use. I suggest that interested readers download two recent (and free) documents on pesticide risk assessment for honey bees:
- The 2008 International Symposium on Hazards of Pesticides To Bees [4], and
- Pesticide Risk Assessment for Pollinators: Summary of a SETAC Pellston Workshop [5]
You may be surprised by how thoroughly every aspect of pesticide testing with regard to bees is being discussed!
Practical application: the regulatory process for risk assessment of pesticides is constantly improving, and is adjusting specifically for the case of systemic insecticides. The regulators are looking long and hard at the neonics [6], but objectively rather than emotionally.
A Balancing Act
Roughly 15% of agricultural crop losses are due to insects, 13% to fungus. Growers call for industry to provide plant protection products to keep them from losing their crops (just as beekeepers call for products to protect our bees from varroa). The plant protection product (PPP) industry tests perhaps 200,000 compounds for any one that it actually brings to market, at a typical cost of some $200 million for each new product [6]. The manufacturers need clear sets of rules in order to maintain the incentive to develop more ecologically-friendly pesticides.
The difficult balancing act between providing the PPP industry with rules, and the well-deserved scientific scrutiny of the effects of manmade pesticides in the environment are handled by regulatory agencies such as EPA and EPPO, with guidance from SETAC and The International Commission for Plant–Bee Relationships.
Practical application: I find it surprising that some advocates keep repeating that the regulatory agencies or the PPP industry are being negligent in looking out for the well being of honey bees—it only takes the slightest bit of homework to see that this claim is entirely untrue!
Who Does the Testing?
In general, after initial in-house testing, a manufacturer will generally shop out “core studies” to an independent lab or university researcher. Some will say that when an independent researcher is paid by the manufacturer to run a trial to test a product, that he is then hopelessly biased. I’ve spoken to a number of researchers, who take great offense at that suggestion! Or manufacturer may hire an independent company to run the trial. At the 2012 Eastern Apicultural Society conference, entomologist Jessica Lawrence from such a company (Eurofin) gave an impressive presentation on the nitpicky details that such a lab must follow in order to meet the highest standards of scientific testing [7].
The EPA even then does not take study conclusions at face value, but has its own reviewers go over them with a fine-toothed comb (for an example see [8]). They then thoroughly analyze all the available data prior to making a registration decision (see the 137-page document for the registration of clothianidin for some crops [9]).
Practical application: The point that I’m trying to get across is that, although the system is not perfect, I tend to trust the EPA’s thorough evaluation of a pesticide more than that of some blogger who has simply read a few abstracts.
Academic versus Field Applicable
Something that confuses the issue is that many of the published studies are academic—of scientific interest, but not necessarily relevant to “real life” situations. The problem with extrapolating from lab tests is that doses which cause adverse effects to individual bees under laboratory conditions may not cause any measurable effect when given to normal free-flying colonies. A number of researchers have told me that there appears to be some sort of colony-level mitigation of the effects of the neonicotinoid insecticides.
EPPO (2012) “adopts the assumption that the most reliable risk assessment is based on data collected under conditions which most resemble normal practice, i.e. by field tests or by monitoring the product in use. Such studies are relatively expensive and difficult to conduct, but the results should be considered as decisive if there is any conflict with results from lower-tier testing (laboratory and semi-field testing)”.
From my point of view, the best perspective is to not get distracted by the hypothetical, but rather to focus upon the two final arbiters of the effects of a pesticide upon colony health—the ability to maintain its population, and the ability to put on surplus honey. These two metrics (cluster size and weight gain) reflect the final calculus of all the potential effects of the pesticide, and are easily measured in the field.
Practical application: this is why I give such weight to the on-the-ground assessment of the effects of seed treatments upon bees by the beekeepers in the Corn Belt and on Canadian canola, who report good colony survival and honey production despite their bees foraging in landscapes with high neonicotinoid use.
Field Relevance
The actual measured amounts of neonic residues found in the nectar or pollen of treated plants are typically in the range of 0-3 ppb (rarely above 5 ppb) (EFSA 2012). However, researchers routinely test bees fed at levels of 25 – 400 ppb, in order to find out what kind of negative effects may occur. The problem is that in many cases, the researchers do not make clear that they are testing at residue levels that would not normally occur under “field relevant” conditions.
The thing to keep in mind is that the neonics are, like nicotine, stimulants. Their effect is similar to that of other stimulants such as the toxic alkaloid caffeine, with which 90% of U.S. adults intentionally dose themselves with on a daily basis.
As an analogy, suppose that you wanted to perform an experiment to determine the effects of the stimulant caffeine on the ability of downhill bicycle racers to negotiate a tricky course. A cup or two of coffee would likely enhance their performance; but imagine if you forced them to drink 10 or 40 cups (still “sublethal doses”) before the race! Would you consider the results to be relevant to everyday real life?
Dr. James Cresswell [10] recently performed a meta-analysis of published research on the effects of neonicotinoids upon bees, in both laboratory and field trials. He then fitted dose-response curves to the data (Fig. 3), which suggested that there would be little expected bee mortality at field relevant doses (the paper is a free download, and worth reading).
Figure 3. Neonicotinoids are typically tested at doses higher than those to which bees would be normally exposed in the field via nectar or pollen. This is a legitimate method for identifying potential negative effects, but the results may not necessarily be relevant under field conditions. Graph roughly after Cresswell 2010.
On the other hand, Cresswell found that “Dietary imidacloprid at measured levels in nectar from two widespread crops is expected to reduce performance [e.g., navigation] in honey bees by between 6 and 11% (oilseed rape) and between 14 and 16% (sunflower). These findings raise renewed concern about the impact of systemic neonicotinoids on honey bees that forage in agriculturally intensive landscapes.”
However, we must again compare those hypothetical performance reductions with reality—colonies in Canada make great honey crops on treated canola, as can bees in areas of treated corn and soy. The problem in reconciling these disparate reports is that several factors come into play in the field:
- The dose makes the poison—field doses from seed treatments are typically (except in the case of planting dust) very low. They are intentionally designed to be so.
- Bees metabolize neonicotinoids quickly [11], similar to the manner in which humans quickly metabolize nicotine, so that they appear to tolerate small doses well.
- Bees appear to find neonicotinoid residues distasteful [12], and avoid drinking highly contaminated nectar. However, they may well bring home highly contaminated pollen or dust.
- Just because an insecticide goes systemic in a plant, that doesn’t mean that bees are constantly exposed to that product. Treated plants only produce contaminated nectar or pollen for a relatively short period of time each season. The rest of the season the bees would ignore those plants.
- Several surveys of trapped pollen found that bees in agricultural areas often mainly collect pollen from plant species other than the treated crops. These findings suggest that bees may be avoiding the treated crops, and that nectar and pollen from the untreated plants would tend to dilute the insecticide residues. However, if the treated crop is the only plant in bloom, then the colony would be exposed to a greater degree (note, however, that colonies foraging on virtually undiluted treated canola appear to do fine).
- The above factors would lead to the dilution of the insecticide within the hive.
- Then there is the “colony effect.” Even when fed extremely high doses of imidacloprid over a period of weeks or months, colonies may continue to thrive (Pettis 2012; Lu 2012; Galen Dively, pers comm).
- This is not to say that exposure to high levels of planting dust can’t result in sudden loss of a large portion of a colony’s adult population!
Practical application: Just as drinking a couple of cups of coffee a day won’t hurt you, a little bit of neonics in the diet don’t appear to harm bees. The question then is always, “How great was the dose?” With modern analytical equipment, that is an easy question to answer by sampling the nectar, pollen, dust, or bees themselves. It is not hard to pin the problem on a specific pesticide if there is actual evidence, which is why it is so important for beekeepers to report adverse effects, and to make sure that samples are taken for analysis!
Problems in Methodology and Interpretation
To be frank, I find many studies on the neonics to exhibit obvious bias—those from the registrant tend to play down any adverse effects; to the contrary, some other labs are clearly on a mission to prove that neonics are the scourge of bees. Therefore, I find myself reading papers on this subject with an extremely critical eye. As an example of a well-designed and objectively interpreted study, I’ve included an arbitrarily-chosen free download in the references: (Aliouane 2009).
The first tier of testing for adverse effects involves laboratory trials with caged bees. One must keep in mind that the results of these studies must be qualified, in that it is difficult to duplicate the natural hive environment and social milieu with a handful of queenless, broodless bees in an incubator, so the results may not really apply to bees in real life. Dr. Geoff Williams has compiled a list of suggestions for the standardization of cage trials, soon to be published.
There are also inherent problems with tunnel and field trials, since it then becomes much more difficult to control extraneous variables, such as the impact of confined flight, weather, alternative forage, disease, and the finding of matching control plots. Often, unforeseen problems (Murphy’s Law applies in scientific research) crop up during a study and the study is junked; in other cases, the researcher openly discusses the problems; but sometimes obvious problems are simply ignored in the write up. Here are a few of the typical questionable details that I see in studies:
- I’ve already mentioned excessive dosing. Exaggerated dosing may help to point us toward avenues for further research, but should not necessarily be interpreted as having any field relevance. I suggest that you take a look at the dosing level in any study. Anything over 5 ppb in feed is likely not relevant to normal field exposure.
- Decourtye [13] found that there were substantial differences in susceptibility between winter and summer bees. There may well also be race and patriline differences to be accounted for.
- Lack of a “positive control”–that is, a dose of a known toxicant (typically the insecticide dimethoate) for comparison. Without a positive control, you really don’t know how the effects of the tested product compare to those from a generic chemical stressor (such as a hive miticide or a natural plant toxin). Amusingly, I’ve spoken with researchers who included a 100 ppb dose of imidacloprid expecting it to be a positive control that would kill most of the bees, but found to their surprise that there was actually little effect at the colony leve!.
- Additional solvents–some labs routinely use the solvent DMSO to first dissolve the neonicotinoid. When I checked with a toxicologist, he said that DMSO is dangerous to even have in a lab, since it greatly increases absorption of chemicals across membranes. Since the bee gut membrane is an effective barrier to neonicotinoids [14], I find such use of DMSO, which is not found in commercial neonic formulations, to be potentially problematic.
- Test bees are often knocked out with CO2 or chilled on ice for easier handling. Both stresses can affect be behavior and survivability [15, 16].
- Lack of control of stress due to parasites. Bees stressed by nosema or virus infection may be more susceptible to pesticide toxicity [17], indicating that in any testing of pesticides, the parasite load of the subject bees should be controlled for.
- Improper incubation temperature of bees or brood. Bee behavior and longevity can be strongly influenced by incubation temperature [18, 19], yet in some studies, the test bees have been severely chilled.
- Running tests solely on very young adult bees, rather than mixed age workers.
- Lack of proper nutrition for caged, newly-emerged bees. Many trials start with bees that are emerged into a near-sterile environment. These “teneral adults” are generally deprived of the normal meal of jelly from a nurse bee (and the included inoculum of the critical endosymbiotic gut bacteria), nor are they fed beebread, or any other protein source. DeGrandi-Hoffman [20] demonstrated that young bees deprived of protein get hammered by DWV. DWV can strongly affect bee brain function.
- In one widely-cited study, it appears that the researcher unknowingly starved the bees for sugar, yet claimed that their mortality was due to the insecticides [21].
- Caged bees are generally not exposed to the normal queen and brood pheromones of the broodnest. We have no idea how such deprivation affects their behavior, physiology, or resistance to insecticides.
Imagine that if we wished to determine the effects of a pesticide on humans, but that we used as test subjects young children that had been ripped away from their families, chilled, starved, and held in isolation, then knocked out and revived, dosed with a stimulant and then watched to see how well they performed some arbitrary test. Would we feel that the results of such a test were applicable to the human community in the real world? Again, the question on any scientific study on bees is whether the results are field relevant.
Practical application: when I carefully scrutinize scientific papers, I find that a number suffer from (often inadvertent) flaws in methodology, or from overreaching interpretation of the results. Luckily, the majority of researchers are meticulous and methodical, and I am greatly impressed by their diligent work! Unfortunately, most beekeepers can’t take the time to sort the good from the questionable.
Recent Studies
There have been several widely cited studies released in the last couple of years—I’ve indicated in the references those that are free downloads. For those few of you who still trust my judgment and objectivity, I’ll give short summaries. Please note that I’ve often corresponded with the authors to get further details of their studies—in general, the researchers are happy to discuss their methodology and findings.
Nosema: Alaux (2009); Vidau (2011); Pettis (2012)—There is every reason to expect a synergy between insecticide stress and nosema infection; neonic treatment may either increase or decrease spore production, but appears to increase mortality in infected bees. However, such results may not be apparent at the colony level. In the Pettis study, after 10 weeks of feeding colonies pollen patties spiked at 5 or 20 ppb imidacloprid, “there was surprisingly no relationship between Nosema infection and imidacloprid treatment which would have been predicted by the lab study.”
Chronic toxicity: Tennekes (2010a, b)—I discussed the paper and his alarming book at length with Dr. Tennekes. He points out legitimate concerns about high levels of residues in surface waters; however, the applicability of the Druckrey–Küpfmüller equation does not stand up to scrutiny, nor does his bird data.
Guttation fluid: Hoffmann (2012) found the guttation fluid droplets on treated melons could contain high levels of neonics. I corresponded with the author about his three studies in Arizona—alternate water sources were available, and he did not observe bees taking up the guttation droplets.
Imidacloprid and CCD: Lu (2012). I don’t wish to belabor this paper’s shortcomings (see ScientificBeekeeping.com for detailed questions). Scott Black, executive director of the Xerces Society for Invertebrate Conservation, called the study “fatally flawed,” both in its design and its conclusions [22]. However, there were two clear conclusions that could be drawn from the study—(1) feeding colonies for four straight weeks with a half gallon of HFCS spiked with imidacloprid at field-realistic levels did not have any negative effects, and (2) then feeding the colonies with sky-high levels of the insecticide for another nine weeks straight still did not harm them enough to cause mortality during treatment or for three months afterward.
Planting dust: Krupke (2012) contained little new information–planting dust can cause bee mortality; the test colonies recovered (Greg Hunt, pers comm). Points out potential synergies with fungicides—there are also other pesticides in the dust. There is a large body of research already published on this issue—see Krupke’s or Marzaro’s (2011) references sections.
Bumblebees: Whitehorn (2012) found that bumblebee colonies fed realistic doses of imidacloprid gained less weight and produced fewer queens. This finding is of great interest, since solitary- and bumblebee colonies are more likely to be affected by pesticides than would be honey bees (due to the population reserve in the honey bee colony). This is of special concern, since native pollinators are already suffering greatly from habitat disturbance and introduced pathogens. “However, it is uncertain as to what extent the exposure situation in the study is representative to field conditions since bumblebees would need to forage for two weeks exclusively on imidacloprid-treated crops in order to be exposed to the same extent as in the study” EFSA (2012).
Homing ability: Henry (2012) glued RFID chips to foragers, fed them a substantial dose of thiamethoxam, released them up to a km away from their hives and recorded whether they made their way home. They then calculated that colonies should crash due to loss of foragers—a result not substantiated in, say, canola fields. Schneider (2012), using similar tracking chips, found that field-realistic doses of imidacloprid or clothianidin had no effect the number of foraging trips from the hive to the feeder, the duration of these foraging trips, and the time interval a bee spent inside the hive between foraging trips, but that much higher doses, as expected, did cause negative effects. The EFSA review (2012) states that “it should be noted that there are several uncertainties regarding these results, therefore, they should be considered with caution. In particular, in the studies from Henry et al and Schneider et al. bees consumed the total amount of active substance within a relatively short period and not administered over a longer period (i.e. a day). Depending on the substance properties and how fast the substance can be metabolised by the bees, this method of exposure could have led to more severe effects than what may occur when bees are foraging.”
Sucrose responsiveness and waggle dancing: Eiri (2012) found that foragers treated with imidacloprid were less responsive to low sugar concentrations in offered droplets of syrup (intoxicated bees “liked” sweeter syrup). They also found that if bees were fed a 24 ppb (about 10x field realistic) dose of imidacloprid, the next day they performed fewer waggle dances (were they “hung over”?). Again, I must question the relevance of such high doses.
Other studies: I could fill the pages of this magazine several times over with my notes on hundreds of studies and my correspondence with various researchers, as I’ve really been trying to make sense of the neonicotinoids. I wish that I could give you cut and dried answers, but the science is not yet there. I’ll continue my analysis in the next issue…
References
[1] Swift (1969), cited in McGregor (1976) Insect Pollination Of Cultivated Crop Plants. http://www.ars.usda.gov/SP2UserFiles/Place/53420300/OnlinePollinationHandbook.pdf]
[2] AFSSA (2009) Mortalités, effondrements et affaiblissements des colonies d’abeilles (Weakening, collapse and mortality of bee colonies). http://www.afssa.fr/Documents/SANT-Ra-MortaliteAbeilles.pdf. This free download, translated into English, is an excellent overall review of colony mortality in Europe by the French Food Safety Agency.
[3] Pimentel, D. (2001) Environmental effects of pesticides on public health, birds and other organisms. Rachel Carson and the Conservation Movement: Past Present and Future. Conference presented 10–12 August 2001, Shepherdstown, W.V. http://rachels-carson-of-today.blogspot.com/2011/02/environmental-effects-of-pesticides-on.html
[4] Oomen, PA and HM Thompson (Editors) (2009) Hazards of Pesticides to Bees: International Commission for Plant-Bee Relationships, Bee Protection Group, 10th International Symposium. http://www.jki.bund.de/fileadmin/dam_uploads/_veroeff/JKI_Archiv/JKI_Archiv_423.pdf. This document is a “must read” for anyone seriously interested in pesticide risk assessment for honey bees.
[5] Pesticide Risk Assessment for Pollinators: Summary of a SETAC Pellston Workshop http://www.setac.org/sites/default/files/executivesummarypollinators_20sep2011.pdf
[6] Whitford, F, et al (nd) The Pesticide Marketplace, Discovering and developing new product. (Broken Link!) http://www.ppp.purdue.edu/Pubs/PPP-71.pdf]
[8] EPA (2003) Data evaluation record honey bee – Acute oral LD50 test http://www.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-044309_20-Mar-03_d.pdf
[9] EPA (2005) EFED Registration Chapter for Clothianidin for use on Potatoes and Grapes as a spray treatment and as a Seed Treatment for Sorghum and Cotton. http://www.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-044309_28-Sep-05_a.pdf
[10] Cresswell, JE (2011) A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology 20(1):149-57. Epub 2010 Nov 16.
[11] Suchail, S, et al (2004) Metabolism of imidacloprid in Apis mellifera. Pest Manag Sci 60:291-296.
[12] DEFRA (2007) Assessment of the Risk Posed to Honeybees by Systemic Pesticides, PS2322. Central Science Laboratory, (GB). http://randd.defra.gov.uk/Default.aspx?Menu=Menu&Module=More&Location=None&Completed=0&ProjectID=13502 This is a long download, but full of good data.
[13] Decourtye, A (2003) Learning performances of honeybees (Apis mellifera L) are differentially affected by imidacloprid according to the season. Pest Manag Sci 59: 269-278.
[14] Suchail, S, et al (2004) In vivo distribution and metabolisation of 14C-imidacloprid in different compartments of Apis mellifera L. Pest Manag Sci 60(11):1056-62.
[15] Ebadi, R, et al (1980) Effects of carbon dioxide and low temperature narcosis on honey bees Apis mellifera. Environmental Entomology 9: 144–147.
[16] Frost, E (2011) Effects of cold immobilization and recovery period on honeybee learning, memory, and responsiveness to sucrose. Journal of Insect Physiology 57: 1385–1390.
[17] Vidau C, et al. (2011) Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE 6(6): e21550. doi:10.1371/journal.pone.0021550
[18] Tautz, J, et al (2003) Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc Natl Acad Sci 100(12): 7343–7347.
[19] Medrzycki, P, et al (2010) Influence of brood rearing temperature on honey bee development and susceptibility to poisoning by pesticides. Journal of Apicultural Research 49(1): 52-59
[20] DeGrandi-Hoffman, G, et al (2010) The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). Journal of Insect Physiology 56: 1184–1191.
[21] Schmuck, R (2004) Effects of a chronic dietary exposure of the honeybee Apis mellifera (Hymenoptera: Apidae) to imidacloprid. Arch. Environ. Contam. Toxicol. 47: 471–478.
[22] Nordhaus, H (2012) The honeybees are still dying. http://boingboing.net/2012/05/07/the-honeybees-are-still-dying.html
Alaux C, et al (2010) Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ Microbiol 12:774–782. http://www.prodinra.inra.fr/prodinra/pinra/data/2011/03/PROD20116cf9b1b_20110315103742504.pdf
Aliouane, Y, et al (2009) Subchronic exposure of honeybees to sublethal doses of pesticides: effects on behavior. Environmental Toxicology and Chemistry 28 91): 113–122. This is a free download, and an excellent example of a rigorous and meticulous investigation into the sublethal effects of some insecticides, in which they freely admit to some surprising and unexplained negative results for thiamethoxam. http://cognition.ups-tlse.fr/productscientific/documents/papers/Aliouane%20ET&C%2008.pdf
EFSA (2012) Statement on the findings in recent studies investigating sub-lethal effects in bees of some neonicotinoids in consideration of the uses currently authorised in Europe. http://www.efsa.europa.eu/fr/efsajournal/doc/2752.pdf
Eiri, D and JC Nieh (2012) A nicotinic acetylcholine receptor agonist affects honey bee sucrose responsiveness and decreases waggle dancing. The Journal of Experimental Biology 215: 2022-2029.
EPPO (2003) EPPO Standards: Environmental risk assessment scheme for plant protection products, Chapter 10, Honeybees. OEPP/EPPO Bulletin 33: 99–101. http://archives.eppo.int/EPPOStandards/PP3_ERA/pp3-10(2).pdf?utm_source=archives.eppo.org&utm_medium=int_redirect
EPPO (2010) Environmental risk assessment scheme for plant protection products. Chapter 10: honeybees. http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2338.2010.02419.x/pdf
Henry, M, et al (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336 (6079): 348-350.
Hoffmann, EJ and SJ Castle (2012) Imidacloprid in melon guttation fluid: a potential mode of exposure for pest and beneficial organisms. J. Econ. Entomol. 105(1): 67-71.
Krupke CH, et al (2012) Multiple routes of pesticide exposure for honey bees living near agricultural fields. http://www.plosone.org/article/info:doi%2F10.1371%2Fjournal.pone.0029268
Marzaro, M, et al (2011) Lethal aerial powdering of honey bees with neonicotinoids from fragments of maize seed coat. Bulletin of Insectology 64 (1): 119-126. http://www.bulletinofinsectology.org/pdfarticles/vol64-2011-119-126marzaro.pdf
Pettis, JS, et al (2012) Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 99(2):153-8. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3264871/?tool=pubmed
Schneider CW, et al (2012) RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0030023
Tennekes, H (2010a) The significance of the Druckrey–Küpfmüller equation for risk assessment—The toxicity of neonicotinoid insecticides to arthropods is reinforced by exposure time. Toxicology 276(1):1-4.
Tennekes, HA (2010b) The systemic insecticides: a disaster in the making. Weevers Walburg Communicatie.
Vidau C, et al (2011) Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. http://www.plosone.org/article/info:doi%2F10.1371%2Fjournal.pone.0021550
Whitehorn, P, et al (2012) Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336 (6079): 351-352.