The Primer Pheromones and Managing the Labor Pool – Part 3
The Primer Pheromones and Managing the Labor Pool
Part 3
Randy Oliver
ScientificBeekeeping.com
First published in American Bee Journal June 2010
I left you last month in the middle of explaining the current model for colony workforce allocation, and the influence of various primer pheromones. Please allow me to pick up where I left off. (Having trouble hitting the keys with my cold fingers—I’ve just pulled off my wet coveralls, and am trying to revitalize myself with hot coffee; and I’m distracted by the annoying buzz of bees in the lampshade as they find their ways out of my clothing).
Brood Pheromone (BP)—the Power of Baby Talk
I’ve already explained that the queen, the larvae, and newly emerged and older workers all beg for jelly from the nurse bees. That raises a fundamental question: Why do the nurse bees give it to them? In order to answer this question, we can again go back to the ancestral model, in which early social wasp larvae secreted an irresistibly alluring saliva and/or an attractive oily substance from their skin, thereby enticing their sisters to feed them. Fast forward to the honey bee–the hungry brood of that society secrete pheromonal component(s) that induce the nurse bees to offer them precious jelly.
However, this simple description does injustice to the complexity of brood pheromone. BP consists of at least ten components, identified by Dr. Yves LeConte in 1990. Since then, others (notably Dr. Tanya Pankiw) have more thoroughly investigated the nuances of BP. The proportions of the components change with the larva’s age, degree of hunger, and needs. BP from young larvae screams for them to be fed (Huang & Otis 1991); from propupae it begs for workers to cap them over.
The above effects are from BP’s role as a short-term releaser that elicits an immediate response from nurses; however, BP has much larger and important function as a primer pheromone throughout the hive (Fig. 1). The presence of BP tells the colony the location and status of the broodnest, and indirectly, acts as a measure of the fertility of the queen. BP stimulates the activation of the nurse bees’ hypopharyngeal glands, and depresses the level of JH in their bodies (likely due to increasing Vg levels) (LeConte 2001)—thus keeping the nurses “young” and producing jelly. BP also affects the older foragers—its presence stimulates them to forage for the pollen necessary for the nurses to produce brood food.
Practical app: although queenless colonies will collect and store pollen, active pollen foraging by the foragers generally indicates that the colony is queenright, and is rearing brood. This is an important factor when renting colonies for pollination—the hives must contain enough open comb for expanded broodrearing and pollen storage. I will return to the potential uses of synthetic BP later in this series.
Figure 1. (This is the same figure as in Part 2 of this article). A simplified diagram of the transfer and effects of primer pheromones upon bee physiology, “aging,” and task behavior; and colony reproduction and wintering. Note the overriding importance of fresh pollen income, and the multiple effects of brood pheromone and queen pheromone. Also note the “push/pull” on mid-aged bees to maintain a balance between foragers and food processors.
Update Aug 2013 An excellent study on the interplay between two pheromones produced by brood– “brood ester pheromone” (non volatile, produced mainly by older brood) and E-β-ocimene (volatile, and produced mainly by younger brood) is that of:
Maisonnasse A, Lenoir J-C, Beslay D, Crauser D, Le Conte Y (2010) E-β-Ocimene, a Volatile Brood Pheromone Involved in Social Regulation in the Honey Bee Colony (Apis mellifera). PLoS ONE 5(10): e13531. doi:10.1371/journal.pone.0013531 http://www.plosone.org/article/info:doi/10.1371/journal.pone.0013531
Another piece to the puzzle
This field of research is advancing so rapidly, that I can now clarify the asterisk that was in my graphic in Part 1—the one that indicated that nurse bees could become diutinus (long-lived) bees only if they had never reared brood. That assumption, based upon Mattila’s (2007) work, was that the physical stress of broodrearing wore out nurse bees such that they could no longer transform into long-lived diutinus bees.
A recent paper by the prolific Amdam team (Smedal 2009) clarifies the actual mechanism. I wish that the paper was more readily available, since it is a great example of good experimental design. Allow me to quote from the paper: “As brood rearing and nurse load decline in colonies toward the end of summer, so does the amount of brood pheromone. Previous work that explains diutinus bee development as a function of brood rearing does not fully account for this fact. To resolve how the amount of brood in honeybee colonies can affect worker lifespan, it is necessary to decouple the effects of nurse load and brood pheromone.”
Smedal “decoupled” the effects of actual brood feeding, vs. the mere presence of BP, by creating small colonies that represented all four possible combinations of the presence or absence of actual brood or synthetic brood pheromone. She then measured the resulting fat body Vg storage levels of young, nurse, and mid-aged bees. Her results were surprising! The presence of BP alone suppressed the storage of Vg in mid-aged bees, whether or not they actually fed brood!
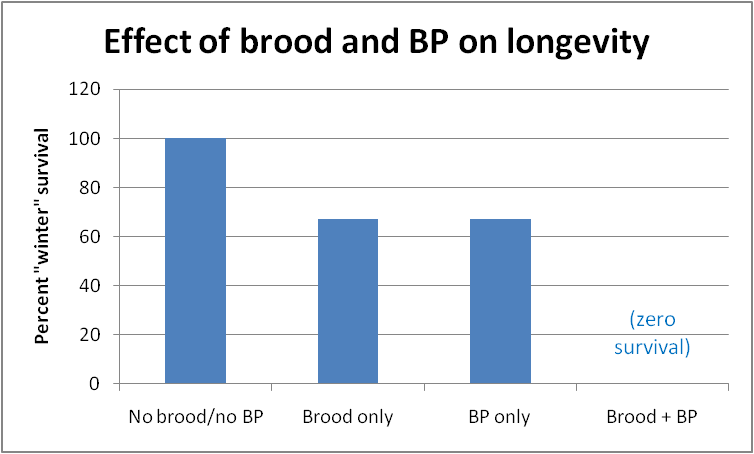
She then placed the colonies into a refrigerated room to mimic winter conditions. No brood had been allowed to emerge in them, so all bees were at least 38 days old when “winter” started. In this case, the addition of actual broodrearing did exhibit an effect—the best survival was in those colonies that neither reared brood, nor were exposed to synthetic BP. The poorest survival was by those colonies that both reared brood, plus were exposed to additional BP (Fig. 2).
Fig. 2 Survival of small (~3000 bees) broodless colonies at 200 days in a 0°C (32°F) room, after exposure to either open brood, synthetic brood pheromone (BP), or lack thereof. Bees that were never exposed to brood or BP lived the longest; those exposed to both, the shortest (all bees were at least 38 days old when the colonies were put into the refrigerator). Note that exposure to synthetic BP alone prevented workers from becoming long-lived diutinus bees. After Smedal, et al. 2009.
The powerful effect of BP on the ability of mid-aged bees to store Vg in their fat bodies helps to explain why bees that have reared brood are unable to live through the winter–it is not only the “stress” of feeding brood that prevents nurse bees from turning into long-lived “winter bees”—it appears that the mere exposure to brood pheromone shortens their lives! Or, conversely, lack of exposure to BP lengthens their lives—this would clearly be an adaptive survival mechanism for colonies unable to rear brood due to lack of forage.
Please note, however, that exposure to BP or brood is not an absolute on/off switch. Harris (2008, 2009) found that workers that emerged in colonies that were rearing a small amount of brood throughout the winter still became long-lived bees, despite some exposure to brood and BP. So this BP/short-lived bee connection seems to be more of a trend, rather than a hard rule. The critical difference appears to be the physical contact of workers with the brood—in a winter cluster, the lesser degree of bee movement, coupled with the existence of only a small patch of brood, apparently prevents most of the winter bees from having their longevity affected by exposure to brood pheromone. More research needs to be done to clarify these effects.
Practical app: The take home message for beekeepers is that essentially, it’s the remaining sealed brood left once the queen shuts down in fall, plus any bees that emerge during winter, that become the long-lived overwintering bees.
This fact is supported by Mattila’s data—nearly all the long-lived “winter bees” developed from eggs laid in the last three weeks of broodrearing. I cannot overstate the importance of this fact to those wishing to overwinter strong colonies! The last round of brood is critical.
Harris (2008), in Manitoba, found that “overwintering populations were defined by the amount of brood reared after 31 August.” He also noted that “When there was a strong honey flow, honey and nectar was stored in the brood areas as quickly as the new workers emerged from them. Once this honey was stored within the brood nest, its storage became permanent when it was in excess of the immediate needs for brood rearing within the brood nest, effectively reducing cell availability for egg laying.”
Practical tip: Do not allow your colonies to become honeybound in late summer, as this condition may not allow the colony to rear enough “winter bees.”
Ethyl Oleate—Forager Feedback
The pioneers in worker-worker interactions were Drs. Zachary Huang and Gene Robinson (both still contributing groundbreaking research). In 1992 they published a seminal paper showing that behavioral development of individual bees was strongly influenced by the number and ages of the bees with which they interacted.
In 1996, in a series of three elegant experiments on small colonies, they demonstrated the incredible physiological plasticity of workers. In their first experiment, they removed all the foragers from the colony. Such removal accelerated the behavioral development of the remaining mid-aged bees into foragers.
In their second experiment, they confined the foragers to the colony by using artificial rain. By forcing the foragers to “hang out” in the hive, they depressed the normal development of mid-aged bees into foragers.
In their final experiment, they removed all the nurse bees, in the absence of brood. Within hours, JH levels in both mid-aged and forager bees dropped substantially, and within two days the hypopharyngeal glands of the older bees that had “reverted” to nurse behavior (inspecting brood cells) had increased in size to 2.5x their original diameter (that’s over 6 times the volume).
Practical application: caged packages may contain mostly nurse bees or a mixture of young and old bees, depending upon how they were shook. Once hived, older bees are able to revert to nursing duties. A similar situation occurs when you leave a weak hive to pick up “drift” when you move a yard of bees out during daylight, or when you pull brood and nurses to prevent swarming. The drift bees will be mostly older foragers, but they will revert to a balanced population in the catch hive.
#2: Yes, it is possible to raise queens with older workers as cell builders, but there is a lag time before they can activate their hypopharyngeal glands in order to produce jelly. This lag will affect the critical initial feeding of the queen larvae.
Clearly, there was some signal that foragers were passing down to younger bees that affected their transition to foraging status. Further experiments to track down the elusive signal concluded in a paper by Leoncini (2004) and an all star cast of researchers (including Huang and Robinson). They identified the forager pheromone that suppresses young bee maturation as ethyl oleate (EO), which, since it elicits physiological changes, would be classified as the third demonstrated bee primer pheromone (along with BP and QP).
Returning foragers produce the pheromone ethyl oleate (EO) in their crop. By passing EO to younger bees, they signal whether mid-aged bees should transition to forager status. This feedback mechanism regulates the efficient allocation of older workers to appropriate tasks.
Of note is that EO is also a component of both brood and queen pheromone. I suspect that EO might function as a “feed me” signal, since it is produced at a high level by the youngest larvae, even higher by the queen, and by the jelly-begging foragers (I can’t find any study that has looked for it in drones). This may be the case, but I haven’t found any direct supporting evidence. The feeding signal is a critical component of bee society, and I’m actually a bit surprised that it has not been investigated more thoroughly (I’m not sure whether foragers and drones beg food simply by antennal signals, or by production of a pheromone).
In any case, EO is again a multifunctional pheromone. Slessor, Winston, and LeConte (2005) coin a new term to describe the effects of the fatty acid esters: “colony pheromones.” High titers of EO and BP in the colony, produced by the queen, larvae, and foragers, serve to keep vitellogenin levels in the nurse and mid-age bees high, and to keep the colony in the “growth” phase. Low levels, on the other hand, shift the colony into “survival” mode.
The adaptive response of mid-aged bees to EO feedback from foragers is beautiful in its simplicity. It works both ways. Forager behavior is modulated by how eagerly mid-aged bees receive their nectar loads—the quicker the unloading, the more stimulated they are perform waggle dances about the source. Conversely, mid-aged bee graduation into foraging behavior is inhibited by the EO that they receive along with that nectar (remember, foragers produce EO in their crops) (see the blocking effect in Fig. 1).
However, when foragers are lost due to the wearing out of their wings or flight muscles, or to the other rigors of foraging, such as drift, risky weather, pesticides, predation, or disease, the EO suppression of forager recruitment from the labor pool is lifted, and more bees can quickly take their places. So how about during a major honey flow? Under that circumstance the colony wants to strike a balance between the proportion of foragers to storage and processing workers. I’m guessing that EO dilution due to the intense nectar flow may help to recruit more foragers.
On the flip side, between blooms (or during rainy weather), when the foragers hang out waiting for scouts to mobilize them, their EO feedback prevents mid-age bees from shifting to forager physiology. As you may remember from my “Old Bees” series, this is important, since bees don’t start “aging” until they shift to foraging behavior (this observation is strongly supported by Harris 2010). So by preventing the mid-aged bees from shifting, the colony maintains a ready reserve force of relatively long-lived workers just waiting for something to do.
As you might have guessed, I still have questions. I’m curious as to what the effect is of the sudden loss of foragers when a colony enters winter. Some heroic and meticulous field work by Lloyd Harris (2008) indicates that broodrearing ceased at exactly the same time that the last of the summer foragers died off–coincidence or not? Furthermore, his colonies initiated midwinter broodrearing just as the “winter bees” started reaching about 84 days of age.
*****************************************
Update June 20, 2012
Independent studies by Heather Matilla and Gro Amdam (references elsewhere on this website–sorry, have to get to the bee yards) demonstrated that the “fall turnover” of the population is due to the lack of incoming pollen, and thus the curtailment of broodrearing. Any bees that were ever exposed to BP leave the hive at this time, causing a major population drop. Any emerging brood is not exposed to BP, and those bees then become diutinus (“winter”) bees.
Re: ethyl oleate, two fantastic papers by Carlos Castillo, et al have helped to explain the dynamics of nurse to forager transition. They explain it thus (see their Fig. 7) in 2012b:
Newly emerged workers stay in the brood nest, cleaning cells and tending the queen. At approximately
1 week, workers develop into nurses that specialize on tending the brood. Young brood require large amounts of food, so these larvae emit (E)-b-ocimene [young BP] which stimulates the nurse-to-forager transition.
Old brood requires less food and more attention from nurses, so these larvae emit BEP [old BP] which delays the transition. In addition, bees throughout the nest are exposed to QMP which also delays the transition .
As nurses age, they move away from the brood nest, to the food storage areas on the periphery. There, the pre-transition nurses interact with the incoming foragers, receiving the food loads. During this close contact, the pre-transition nurses become exposed to the EO that is on the forager’s cuticle and that delays the onset of foraging in the nurses .
The transition from nursing to foraging is preceded by a burst in the juvenile hormone III level, and this burst triggers the transition . The transition is accompanied by many changes in the behavior and physiology of workers. One such change is the ability to convert ethanol, which is present in flower nectar, to EO and to exude the EO via the cuticle.
Castillo, C, et al (2012a) Biosynthesis of ethyl oleate, a primer pheromone, in the honey bee (Apis mellifera L.). Insect Biochemistry and Molecular Biology 42: 404-416.
Castillo, C., et al. Seasonal variation in the titers and biosynthesis of the primer pheromone ethyl oleate in honey bees. Journal of Insect Physiology (2012), http://dx.doi.org/10.1016/j.jinsphys.2012.05.010
Castillo (2012b) further explains the pheromone dynamics:
The nurse-to-forager transition is influenced by other pheromones as well. Brood ester pheromone (BEP), which consists of a blend of fatty acid methyl and ethyl esters and is produced by older brood, delays the transition (Le Conte et al., 2001, 2006; Pankiw, 2004), but (E)-b-ocimene, which is produced by young brood, enhances the transition (Maisonnasse et al., 2009, 2010a,b). Queen mandibular pheromone (QMP) stimulates pollen foraging, by causing an increase in the number of pollen foragers and their load sizes, during the spring in small colonies, but not during the summer in large colonies (Higo et al., 1992). However, high doses of QMP (as might be experienced when bees are confined to the colony during winter) delay the age at which workers first forage (Pankiw et al., 1998). Environmental conditions, such as temperature (Huang and Robinson, 1995), and pathogens, such as infection with Nosema spp., also alter EO production in workers (Dussaubat et al., 2010) and influence the transition of workers from nursing to foraging.
****************************************
I wondered if the sudden absence of ethyl oleate due to forager die off affects the colony. Apparently, the lack of such in the absence of brood does not cause the promotion of “winter bees” to forager status, perhaps due to the absence of brood or the inability to fly in the cold. So that brings us back to the question of what is the proximate cause for the initiation of midwinter broodrearing?
Could it be that one of the factors involved is the transition of aging “winter bees” to forager status (to prepare to fly out of the hive to die of old age), and thus the production anew of ethyl oleate, which might stimulate the initiation of broodrearing? Could this be a mechanism by which colonies “know” when to start rearing replacement bees? I bounced this idea off the scientists at Contech, and they supplied me some custom pheromone strips to test my hypothesis this winter (thank you!). Results: see my research report in an upcoming issue.
Update: I ran the above trial, and didn’t find any effect of added ethyl oleate upon winter brood production, so the question of what actually initiates winter broodrearing is still unanswered. Perhaps the better question is instead, what causes bees to cease broodrearing?
I’m out of space now, so will continue with queen pheromone, colony reproduction, and the missing pieces in the puzzle.
Quick Status Report
There were major collapses of colonies in some operations this winter. Many collapses were predictable, as called early by Lyle Johnson and others, due to the poor weather and forage conditions in much of the country. Many colonies went into winter in poor nutritional shape, exacerbated by early frosts that killed off some fall pollen sources, such as Rabbitbrush. A number of beekeepers put off mite treatments in order to get that last super of honey, and paid the price later as mite populations increased past the point of no return. Finally, unusual winter freezes in Florida and Texas shut down colony buildup during the expected winter nectar flows.
The above are usual and unsurprising suspects for colony collapses—poor nutrition, too many mites, unexpected chills. However, CCD appears to have raised its ugly head again, with some good beekeepers having their operations decimated by sudden losses of the adult bee population. I’m hoping that samples taken by researchers who visited California may narrow down the actual cause of CCD. (Here’s a teaser–a recent field trial that I performed sure points the finger at a virus or virus/Nosema ceranae synergy. I will report on this later).
Too many spores! This sample is running at about 50 million spores per bee (calculated by taking the average number of spores per square and multiplying by 4 million). This sample of bees is clearly in trouble. The N. ceranae/virus/poor nutrition combination may be fatal to colonies.
Speaking of N. ceranae, a number of beekeepers are finding high levels this spring, despite the colonies being very strong. My own operation falls into this category—spore counts were up on my truck net samples coming back from almonds, but the bees are the best I’ve seen in years, and I’m too busy splitting them to keep them from hitting the trees to even test for nosema!
Beekeepers are asking whether they should treat. Well first, I don’t give advice—I just report on the biology, and leave it to you to make business decisions. Here are some facts: we know that N. ceranae has been present in East Coast operations for at least 25 years, and didn’t appear to be a particular problem. It was also strongly established in California operations when we first looked for it in 2006, sometimes at high levels in very strong and healthy-appearing colonies. However, some of us had unidentified collapses and poor buildup in 2004-2005, but I’m hesitant to blame them fully on nosema, since my untreated N. ceranae test colonies generally did just fine.
So here’s the problem: we simply don’t have historical data to see what kind of baseline N. ceranae count is “normal” in the spring. No one much looked for nosema in the spring—so N. ceranae could have been there for a long time, but no one noticed. Now that we’re looking for it, we are finding it, but we really don’t know what to make of it.
Data from all over North America indicate that N. ceranae spore counts tend to spike in May and perhaps in fall, similar to those of N. apis. Counts typically drop in June, whether you treat or not. Counts may climb during winter in warm areas.
Some beekeepers report that colonies are set back by spore counts of only a few million average in field bees, yet others tell me about booming hives exhibiting higher counts, and that treatment with fumagillin doesn’t bring counts down. It appears that colonies can handle the parasite fairly well if there is good pollen nutrition. At what level treatment is necessary or cost effective, I’m not sure that anyone can really give you a good answer.
It may be the case that colonies can handle N. ceranae fairly well as long as they aren’t dealing with other stresses, such as wet weather, poor nutrition, high mite levels, or especially the wrong viruses. This puts many beekeepers who have not normally used nosema treatments (this is the category into which I fall) in a quandary—should we be cautious and treat, or take our chances and see if the bees ride it out? I sure don’t have the answer!
References
Harris, JL (2008) Effect of requeening on fall populations of honey bees on the northern Great Plains of North America. Journal of Apicultural Research and Bee World 47(4): 271–280.
Harris, JL (2009) Development of honey bee colonies on the Northern Great Plains of North America during confinement to winter quarters. Journal of Apicultural Research and Bee World 48(2): 85-90.
Harris, JL (2010) The effect of requeening in late July on honey bee colony development on the Northern Great Plains of North America after removal from an indoor winter storage facility Journal of Apicultural Research and Bee World 49(2): 159-169
Higes, M, et al (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environmental Microbiology 10(10): 2659–2669.
Huang, Z-Y and G. Otis (1991) Inspection and Feeding of Larvae by Worker Honey Bees (Hymenoptera: Apidae): Effect of Starvation and Food Quantity. J. Insect Behavior 4(3): 305-317.
Huang, Z and GE Robinson (1992) Honeybee colony integration: Worker-worker interactions mediate hormonally regulated plasticity in division of labor. Proc. Natl. Acad. Sci. 89: 11726-11729.
Huang, Z and GE Robinson (1996) Regulation of honey bee division of labor by colony age demography. Behav Ecol Sociobiol (1996) 39 : 147–158.
LeConte Y, Mohammedi A, Robinson GE (2001) Primer effects of a brood pheromone on honeybee behavioural development. Proc R Soc Lond B 268:1–6
Leoncini, I., Le Conte, Y., Costagliola, G., Plettner, E., Toth, A. L., Wang, M., Huang, Z., Bécard, J.-M., Crauser, D., Slessor, K. N. and Robinson, G. E. (2004) Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. Proc Natl Acad Sci USA 101: 17559-17564.
Mattila HR, Otis GW (2007) Dwindling pollen resources trigger the transition to broodless populations of long lived honeybee each autumn. Ecol Entomol 32:496–505.
Pankiw, T (2004) Cued in: honey bee pheromones as information flow and collective decision-making. Apidologie 35: 217–226.
Slessor KN, ML Winston, Y LeConte (2005) Pheromone communication in the honeybee (Apis mellifera L.). J Chem Ecol. 2005;31:2731–2745.
Smedal, B, M Brynem, CD Kreibich and G. V. Amdam (2009) Brood pheromone suppresses physiology of extreme longevity in honeybees (Apis mellifera). The Journal of Experimental Biology 212: 3795-3801.