Nosema Ceranae: Kiss of Death or Much Ado about Nothing?
Nosema Ceranae:
Kiss of Death or Much Ado about Nothing?
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in Aug. 2009
Once again I’m interrupting a series of articles to update beekeepers on breaking news that has practical applications in bee management. This time the information is about Nosema ceranae, and I felt that the readers of the Journal might benefit from an update on recent research results.
Dr. Mariano Higes’ team (from the Centro Apicola Regional in Spain) electrified the beekeeping world two years ago with the report that the “new” bee parasite, Nosema ceranae, appeared to be causing massive colony depopulations (Martín-Hernández 2007). Suddenly we had a potential culprit for the cause of CCD! I followed up on this announcement with a series of articles on “The Nosema Twins”—all now posted to ScientificBeekeeping.com. There has been a recent flurry of papers published on N. ceranae (several by the Centro Apicola team—who have produced the largest body of research on the parasite), so I felt it appropriate to update my readers.
My tongue-in-cheek title for this article refers to the diametrically opposed evaluations of the effects of the “new” nosema on bee colonies in Spain. Papers published by Dr. Higes’ prolific team suggest that without antibiotic treatment, any colony that is infected by N. ceranae is eventually doomed. On the other hand, Antonio Pajuelo (Consultores Apícolas) and Jose Orantes-Bermejo (Director Técnico Laboratorios Apinevada) are adamant that little if any correlation exists between N. ceranae infection and colony collapse!
This is more than a mere academic debate, as beekeepers worldwide are forced to make expensive management decisions, including very expensive antibiotic treatment and the sterilization of contaminated combs.
Here in the U.S., some beekeepers blame N. ceranae for taking down their colonies, and depend upon regular use of fumagillin to stay in business, whereas others have yards of mildly infested colonies that thrive and produce great yields of honey (this is the case in my own operation—“Bonehead Beekeepers”—where we vigorously pursue the single- minded quest of exploring every conceivable way to lose money at beekeeping). So what’s a beekeeper to think? Unfortunately, I can’t yet answer that question, but I will try to digest and sort out the current state of knowledge.
The “Nosema Twins” are not twins
They are actually more like cousins. Despite the titles of my previous series on N. ceranae, we have come to find that N. ceranae is more closely related to N. vespula (from yellowjacket wasps) than it is to the “old” N. apis (Chen 2009). This finding has major consequences, in that it tells us that we can’t simply apply what we know about N. apis to N. ceranae—it’s a different critter.
In addition, there are different “strains” (haplotypes) of N. ceranae, which may exhibit different degrees of virulence (N. ceranae is a more “generic” parasite, and can infect various hosts). And if ceranae is killing off colonies, then natural selection is certainly exerting a pressure in each affected area towards the development of resistant bees.
Dr. Denis Anderson explained to me that “Nosema [apis] follows a very much “temperature driven” pathology. That is, a few bees go into winter infected, they spread spores to neighboring bees in the winter cluster (forming ‘pockets of infection’ within the cluster). These pockets get larger toward the end of winter until they are completely eliminated in spring when the infected bees fly out and die. Generally, levels of nosema stay low over summer, until autumn when there is a small peak, and again this is mostly temperature driven.”
Nosema ceranae follows a different seasonality. Instead of spiking only in November and March like its cousin, it is present throughout the year, and thrives in summer. Colonies may struggle or collapse even during a spring or summer bloom.
The two nosemas both infect epithelial cells that line the bee gut, but the similarity ends there. Higes found that N. ceranae goes on to infect the basal cells, and Dr. Chen found that it then apparently travels throughout the entire alimentary tract, including the hypopharyngeal and salivary glands. But only 20% of the fat bodies were infected, and no muscle tissue.
Raquel Martín-Hernández (2009) found that N. ceranae infects bees held at 25°C (77°F) more quickly than does N. apis. She also found that N. apis growth appears to be limited to a narrow temperature range. It grew at 25°C (77°F), which is a bit cool for a bee, thrived at 33°C (91.4°F—on the low side of brood nest temperature), but died off at 37°C (98.6F—typical of warmed bee flight muscles, or perhaps a brood nest with a “fever” (Starks 2000). N. ceranae, on the other hand, is able to survive at 37°C.
The take home message is that N. ceranae is a somewhat more virulent parasite, and apparently more heat-adapted than its cousin. We can no longer count on the warmth of summer (or induced fever in the bees) to make nosema disappear.
Nosema apis is a cold weather disease, evidenced by the symptom of dysentery (above). An infection by N. ceranae is generally without symptoms—the older bees simply “disappear.” Photos by the author.
Infection of Queens
Nosema apis was a nasty problem for queen bees, often causing early supersedure. From my reading of numerous studies, I get the hunch that the parasite is most effectively transmitted from the attendants to the queen during the chilling and stress of shipment or holding at room temperature (Lehnert 1973, Webster 2004). At that point, the queen can then become a “Typhoid Mary”—infecting the workers via the spores in her feces, which are licked up by the attendant bees (Loskotova 1980).
Luckily, with N. ceranae we get a break! It doesn’t appear to transmit readily to the queens, at least until the final stages of colony collapse (Higes, pers comm). This is good news for queen producers, who may experience more trouble at eliminating ceranae than they did with apis.
Immune Suppression
Nosema is tough on individual bees, not least of which because the infection inhibits their ability to digest food. Molonea (1998) found reduced digestive proteolytic activity in young bees infected with N. apis. Dr. Mariano Higes explains that bees infected by N. ceranae simply starve to death in the midst of plenty due to lack of digestive function.
As if induced nutritional stress were not enough, N. ceranae also appears to suppress the bees’ immune functions. Karina Antúnez (2009), found that bees ramp up their immune systems in response to N. apis, but that system is apparently suppressed by N. ceranae. In addition, infection by N. ceranae depresses the level of the bee “fountain of youth,” vitellogenin, suggesting that infection may decrease their lifespan by this effect.
So the poor bees become both nutritionally stressed and immune suppressed—likely candidates for latent viruses to exploit. This is before we even add mite damage and miticide/pesticide stress. Since a nosema also broaches a bee’s main barrier to virus infection—the intact gut epithelium—we can suspect that we’ll find N. ceranae/virus associations.
Just to make things more exciting, Santiago Plischuk (2009) has documented that N. ceranae has infected at least three species of bumblebees in Argentina. I’m not sure of the implications of this, but when a parasite hops between hosts, more virulent forms can emerge.
Changes in Behavior
Perhaps the most noticeable effect of N. ceranae infection is the lack of population buildup of infected colonies, due to the premature death of infected foragers. Of interest is that nosema-infected bees tend to forage at cooler temperatures. Woyciechowski (1998) suggests that infected bees may engage in more risky foraging behavior, perhaps sensing that they do not have long to live. Additionally, infected bees may simply fly off to die, exhibiting an “altruistic suicide” to help prevent the infection of nestmates (Kralj 2006).
Another symptom, reported by several, and described by Bob Harrison on Bee-L, is that of bees not taking syrup, and then massively drowning in division board feeders. Bob feels that the symptom of going “off feed” is a good indicator for N. ceranae infection, which can be reversed with a drench of fumagillin syrup.
The drowning behavior may have an explanation in a recent paper by Chris Mayak (2009), in which he found that “N. ceranae imposes an energetic stress on infected bees, revealed in their elevated appetite and hunger level…. infected bees attempt to compensate for the imposed energetic stress by feeding more…” Mayak suggests that such hungry, nutritionally stressed bees, exhibiting risky foraging behavior, might play a role in the depopulation of infected colonies.
Development of Vegetative Stage/Spores
Most nosema species in other insects infect the larvae. Neither of our nosema “cousins” do so. This is unusual, especially since the adult honey bee has a relatively short lifespan of only a few weeks during summer. That means that the parasite would be expected to try to infect the bee early in its adult life, so that it can go through several generations before the bee dies, in order to multiply and produce an abundance of spores.
The mode of transmission for N. apis is mainly by house bees ingesting spores when they clean “soiled” combs, and perhaps for N. ceranae by eating spores in stored pollen (Higes 2007). Both activities are performed mainly by very young house bees, so you would expect them to quickly become infected.
Curiously, although nosema can complete a reproductive cycle in just a few days, massive sporulation in young bees does not appear to happen. Yefimenko (1994) found that inoculated caged bees did not develop substantial sporulation in the gut until they reached forager age (about 20 days old), despite the age that they were inoculated. Matthew Smart (working with Dr. Steve Sheppard, pers comm) observed the same delay in marked cohorts of emerging bees in N. ceranae infected colonies.
This late development of spores should be apparent to all if one compares the average spore load of house bees to the older field bees of the same colony. The mean spore counts for the older field bees will typically be 10-20 times higher than that of the house bees. Note: bee “age” is more a function of behavior, than of chronology. Bees begin to age once they transition from house functions to foraging (see “Old Bees, Cold Bees” at my website).
So the question in my mind is whether N. ceranae could multiply in the vegetative form in bees without producing spores, and that we might be overlooking actual infection by only looking for spores in the gut contents. This suspicion was bolstered when I spoke with Zita Sanchez, a nosema technician who works for Ken Olley in Australia. Zita previously studied Nosema bombycis, which infects silkworm larvae. N. bombycis can maintain a vegetative infection without producing spores.
A partial answer is given by Raquel Martín-Hernández (2009). She found that N. ceranae, in the early stages of infection, produces more vegetative cells relative to spores than does N. apis. So a lack of spores in the gut does not necessarily mean that the bee is not suffering from the early stages of an infection.
And how about Dr. Chen’s finding of ceranae DNA in the hypopharyngeal glands? Could the vegetative stage be passed along in the jelly shared between workers? There are still a lot of unanswered questions about this parasite!
Transmission of Spores
When a colony dies with N. apis, the combs are generally soiled with brown spotting and streaks from the spore-laden dysentery of the infected bees. This contamination has been clearly demonstrated to infect fresh bees placed back on the combs.
When a bee ingests nosema spores, its digestive fluids cause the spores to release the harpoon-like polar body. In order for a spore to actually infect a bee, the harpoon must get past the protective peritrophic membrane, and penetrate an epithelial cell. Even then, the vegetative body must overcome the innate and induced immune responses of the cell in order to reproduce.
Most spores are not successful. Bees (and humans) are constantly bombarded by spores (you breathe in plenty each day), yet most of us don’t get sick every day! Scientists use the term “ID50”—the infectious dose necessary to infect 50% of the host population. Dr. Ingemar Fries (pers comm) has found that the ID50 for either nosema to be close to 100 spores. Fewer than that, and half the bees won’t get infected.
Once a spore does take hold, the infection can multiply rapidly. The infected cell can release new spores into the gut in 2-3 days, and some of those spores may “autoinfect” the bee, rather than passing out of the gut. The infection can then spread throughout the epithelial layers by day 6, with bees (in the lab) dying on day 7 (from N. ceranae).
But not all spores are created equal. As with “hardened” plant seeds, or the cysts of brine shrimp, some organisms can produce different kinds of “eggs”—some which hatch quickly, and some that stay dormant for a time. Youssef (1971) found that N. apis can produce different types of spores in summer and winter. We have no idea as to whether N. ceranae does the same.
With N. apis, it is pretty clear that transmission is via feces—either due to dysentery of infected bees confined during winter, or from an infected queen (Czekońska 2000). With N. ceranae it was easy to assume that the same would apply. However, in beekeeping, assumptions can lead to erroneous conclusions.
There is no dysentery associated with a N. ceranae infection. So how do the spores get transmitted? Higes found spores in pollen carried to the hive, and demonstrated that it could be infective. But no one has clearly demonstrated the normal means of transmission.
This has important practical implications, since many beekeepers are assuming that they should sterilize combs of deadouts with acetic acid (or by other means) before putting them back to use. At the national conventions this year I asked around to see if any researchers had actually tested combs to confirm that they were contaminated with N. ceranae spores. None had.
It occurred to me that it might be foolish to be going to all the expense and effort of comb sterilization based only upon the assumption that the combs were loaded with spores. So I invited Florida apiary inspector Dave Westerveld to visit my N. ceranae test yard between conferences. I’ll detail our findings in the photos below.
A Quick and Dirty Field Search for Spores
Dave Westerveld using the Suck-a-Bee to confirm the infection level of N. ceranae. We tested combs from an untreated colony that had been infected with ceranae for at least two years at about 5 million spores in the foragers, and from a deadout in which the dead house bees tested at 10M spores.
Nancy Gentry (promoter of the Florida Honey Standard of Identity) helping out at our makeshift field laboratory.
We found that a swab with hot water from a thermos quickly dissolved and lifted residues from the face of the combs and the insides of the cells.
 We swabbed, ground, crushed, and squeezed numerous samples in our search for spores.
We swabbed, ground, crushed, and squeezed numerous samples in our search for spores.
This is a typical view of comb surface residue at 400x magnification, which includes pollen coats, dirt, etc. But the vast majority did not contain any nosema spores whatsoever!
The next day, Dr. Jerry Bromenshenk (sans white lab coat) stopped by to share in the fun. We looked for spores in beebread and honey in the infected colonies. Again, they were few and far between.
A typical view of diluted fresh beebread—a mixture of pollen grains, but again, it was hard to find a single spore.
I reported our findings to Dr. Robert Cramer. He checked a comb that had been sent to him from an N. ceranae deadout, and was able to find spores. So I asked him to send the comb to me so that I could confirm my ability to detect spores by swabbing. I did indeed find spores (so the swab technique works), but not many. He didn’t know the history of this comb, so I’m not sure why there were more spores than in the combs that we tested.
Bottom line: I’m not convinced that there are enough spores on combs of colonies infected with a moderate level of N. ceranae (up to 10M spores/bee) that a young bee would be likely to ingest the ID50 during the normal processes of comb cleaning and pollen consumption. I hope to test this hypothesis once I free up my incubator.
Sterilization of Combs/Viability of Spores
OK, so let’s say that you’re the cautious type, and still want to kill any N. ceranae spores on the combs of your deadouts. Acetic acid vapors and radiation are options, and Dr. Cramer has found that bleach or lye also work.
However, Dr. Cramer reported some very interesting observations when I first supplied him with spores from my yard back in early 2008. He found that when he put the spores into the ‘fridge over the weekend, that a lot of them appeared to die (become nonviable) over the weekend! This was a great surprise, since N. apis spores are routinely frozen for storage, and spores in general survive better when chilled.
Dr. Cramer (unpublished data) analyzed groups of 10,000 spores. They started at about 87% viability. After only an hour in the refrigerator, viability dropped to 70%. After an hour in the freezer, viability dropped to 10-50%. At -80ºC (-112ºF) for an hour, generally only 5% of the spores survived. These are preliminary figures, and he is continuing this line of research.
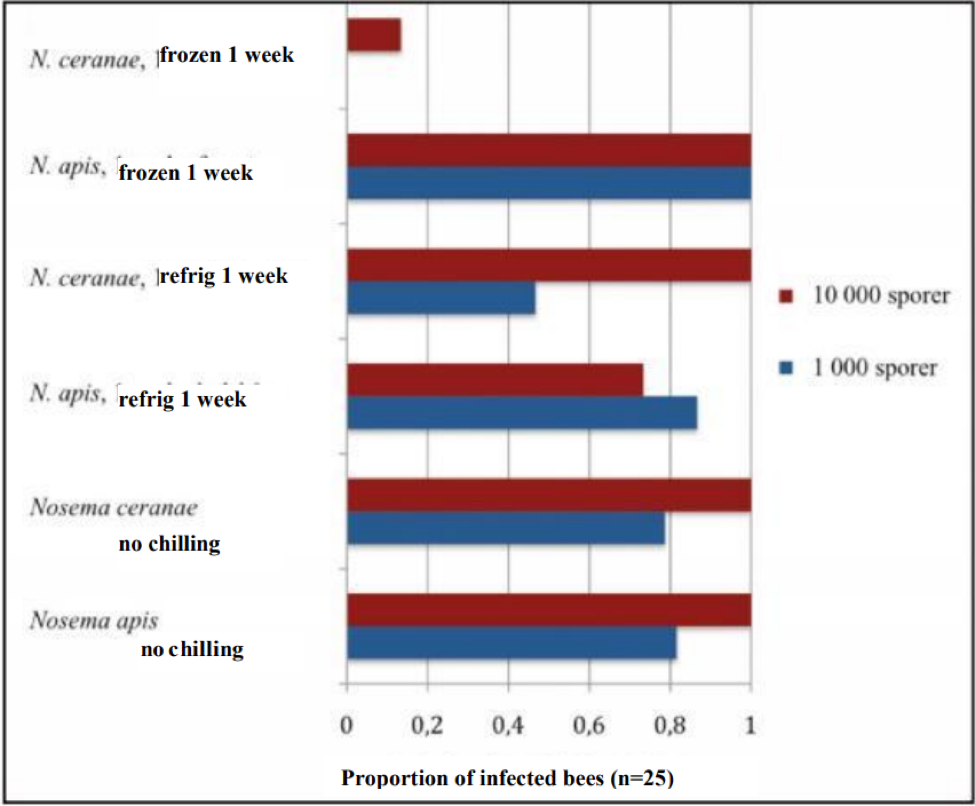
At about the same time, Dr. Ingemar Fries and Eva Forsgren in Sweden also were having troubles at infecting bees with frozen spores. They’ve quite recently (2009) published some stunning similar results (by the way, Google translate does a great job going from Swedish to English). I’ve superimposed English labels onto his graph below.
Effect of chilling on the infectiveness of nosema spores. Unchilled spores of both species (bottom bars) infected 80% of the bees with a 1000-spore dose, and 100% with a 10,000 spore dose. The infectivity of N ceranae spores dropped substantially after a week of refrigeration, and dramatically after a week of freezing. Modified from Fries (Bitidningen 107, 2009) by permission.
Note: The above study has important implications to beekeepers. What is tells you is that in most of the U.S., winter freezing should be enough to kill most of the spores of N. ceranae! This supports the observation of a number of commercial beekeepers that letting deadout equipment “rest” for a month at cold temperatures resulted in better colonies when restocked in the spring.
When I and other researchers first heard of this observation, we scratched our heads, since in general, spores of disease organisms remain viable when stored at cold temperatures (there is normally more degradation at higher temperatures). None of us would have guessed that N. ceranae spores would be so intolerant of cold storage!
A very interesting additional observation by Fries was that N. apis spores actually appear to become even more infective after exposure to freezing! Note in the graph above how the 1000-spore inoculum of N. apis infected 100% percent of the bees after having been frozen—a higher percentage than with unchilled or refrigerated spores! Dr. Fries is planning further investigation into this surprising phenomenon.
The susceptibility of N. ceranae spores to freezing may help to explain why it appears to be more of a problem in warm areas. This point should not be lost on those Midwestern beekeepers who take colonies to California for almond pollination—any deadout equipment there won’t experience the “sterilizing” effect of cold winter temperatures.
So what if you live in a warm climate? You’re still in luck, since ceranae spores appear to be rather fragile. Dr. Cramer also found that they succumb quickly to elevated temperatures, to wit, 120°F for 90 minutes. Cramer hopes to develop a time/temperature curve of spore mortality, so that a beekeeper can determine how much time at what temperature it takes to kill most of the spores.
Thresholds for Treatment
A recent paper by Martín-Hernández (2009) found that both N. ceranae and N. apis can complete their life cycles (spore to spore) in only two days in gut cells, and that N. ceranae initially produced a larger proportion of vegetative cells relative to spores than did N. apis. This finding has profound implications as to the reliability of using spore counts alone as a means of determining how badly a bee (or a colony) is infected. Martín-Hernández suggests, with scientific terseness, that spore counts are “unreliable as a tool to evaluate the health status of infected bees.” Unfortunately, there is no easy or cheap alternative available to the beekeeper. Again, I caution the beekeeper about putting too much faith in spore counts, especially when taken from either inside the colony, or from samples of only a few bees.
The main point to clarify is that there is a difference between being infected by a parasite, and suffering from disease caused by the parasite. Even at a mean spore count (of foragers) of 5M, there are only a very small proportion of the total population of bees in the colony actually infected to any degree. The true concern is when the infection level grows to include a substantial proportion of house bees, at which point the colony will quickly collapse. Sampling of field bees will indicate if the colony is infected. Sampling of house bees may be the best way to determine if the infection is serious.
Perhaps the simplest way to evaluate the effect of N. ceranae on your bees is to monitor how well your colonies are building up and producing (not discounting the lack of interest in syrup). If ceranae is indeed killing off a noticeable proportion of field bees, you will notice that the colony cannot build its population (although Higes describes a “false recovery” by which the colony compensates by massive broodrearing).
In my own test yard, the only colonies that I find that appear to suffer when infected with N. ceranae are those that also have disease of some sort in the brood. There appears to be a (new?) brood disease that has appeared in the past three years, in which the brood pattern goes spotty (cells filled with brood of mixed ages, rather than a nice pattern), the dying larvae turn yellowish, and die with “EFB-like” symptoms. I spoke yesterday with Dr. Jeff Pettis–he and Dennis vanEnglesdorp are also seeing it on the East Coast. They’ve bestowed the descriptive name to it of “snotty brood.” In my own test yards, the disease does not appear to spread easily from colony to colony, and I find that a shot of either antibiotic may help, and that bees can be successfully restocked on to deadouts without treatment. I’m eager for Dr. Jerry Bromenshenk and Dave Wick to figure out how to get brood samples to run through the IVDS machine, to see if they can identify the pathogen(s) involved.
Most researchers in various countries, other than the Higes team in Spain, do not see a strong correlation between N. ceranae spore counts and winter losses. Nosema infection of any sort is clearly a stressor to the colony, and an invitation for viral infections. However, as with most bee diseases, colonies do their best in conditions of good forage and weather. If your yards are crowded or the natural pollen supply is inadequate, or there are mite or other disease issues, then it would be prudent to be more concerned about N. ceranae.
I was recently speaking with a large commercial beekeeper from a warm climate who found that at spore counts of above 2M, his colonies simply did not build. However, another very large operator from Southern California does not even worry about counts in the range of 5M. In my location in the California foothills, counts of 5M do not appear to affect colony production or survivability.
To monitor N. ceranae levels, be sure to collect bees from the entrance. A number of beekeepers have built Suck-a-Bee vacuums (plans at my website). The feedback is that the 16V model is better, since it holds much more charge, and you don’t need a separate battery. If you don’t have a vacuum, you can brush bees off the landing boards into a widemouth jar containing rubbing alcohol (a small piece of screen to block the entrance will make it easier—screen works better than a block of wood). Be sure to collect at least 50 bees in every sample—we suck or sweep a dozen each from several colonies in each yard all into the same jar as “yard samples,” and take them home in labeled jars.
If you don’t have a bee vacuum, you can simply sweep bees off the landing board into a wide mouthed jar of alcohol. Just as you can’t judge the mite level of a yard by sampling a single colony, in order to determine the yard average for nosema, you should take several bees from as many colonies as possible. Be aware that sweeping the entrance really sets the bees off—gloves are a good idea!
Regarding microscopes, over the past year, I’ve had the opportunity to view nosema spores through a number of microscopes—some quite expensive! I’ve yet to see a scope that gives a clearer image of spores than the Omano, which I still feel is the best buy.
Treatments
We’re still struggling to determine the most effective and appropriate treatments for N. ceranae. Large-scale field trials by Drs. Jeff Pettis and Steve Pernal have been bedeviled by the spontaneous disappearance of N. ceranae from the control groups. This inconvenient trait of the parasite forces one to call into question any beekeepers’ reports that any certain treatment was effective, if they didn’t run untreated control colonies alongside the treated colonies.
Fumagillin often works, but not always. It appears to me that one may need to increase the recommended dose by about 25%. N. ceranae may return a few months after treatment.
Researchers and beekeepers are still experimenting with alternative treatments. The strong “cup o’ soup” (8 oz of drench, 454g bottle of Fumagilin B in 7 gal of syrup) gets good feedback. Nosevit appears to work, if applied precisely by label instructions (1/4 tsp in only 12 oz of syrup). Both of these treatments indicate that giving the dose of active ingredient in cups, rather than gallons, of syrup (other than for winter stores) may be more effective.
HoneyBHealthy appears to be somewhat beneficial. I don’t have enough data on the other herbal products, such as Vitafeed Gold, Protofil, and ApiHerb. And I’m still curious to see if I can get thymol to have an effect.
We don’t know why N. ceranae appears to displace N. apis. If a bee is inoculated with both species, both grow (Fries 2009), so there doesn’t appear to be an inhibitory competition. However, treatment with fumagillin may favor N. ceranae.
I caution against treating your bees unnecessarily! Not only can treatments be expensive, but some, if not all, are stressful to the bees.
Bottom Line
1. Nosema ceranae is different than Nosema apis, and exhibits different modes of infection, virulence, epidemiology, and pathology.
2. N. ceranae appears to be more of a problem in some areas and operations than others. It may be more of a problem in warm areas. Fortunately, in some areas, the spore counts never progress above the 4-8M range.
3. It is likely a waste of money to treat if you haven’t confirmed that your bees are indeed infected.
4. N. ceranae appears to only infect queens shortly before the collapse of the colony.
5. Initial signs of infection may be lack of colony growth, due to early death of foragers and nutritional stress, and of going “off feed.” In the later stages, house bees become infected, bees may drown en mass in division board feeders, and the colony may suddenly collapse.
6. We’re not really clear as to how N. ceranae is transmitted from bee to bee.
7. We’re not really clear about the best treatments, or application thereof, or if treatment in general is even necessary or cost effective!
8. Luckily, N. ceranae spores are more delicate than those of N. apis—they are readily killed by exposure to either high or low temperatures.
9. This last point is of major practical application—it may be unnecessary to attempt to “sterilize” combs if they are simply stored at cold (or warm) temperatures.
Acknowledgments
I greatly appreciate the time given to me by researchers Mariano Higes, Antonio Pajuelo, Jose Orantes-Bermejo, Jeff Pettis, Ingemar Fries, and Eva Forsgren. Thanks also to commercial beekeeper Bob Harrison, and especially to Peter Borst for his invaluable assistance in my electronic research.
References
Antúnez, K, Raquel Martín-Hernández, Lourdes Prieto, Aránzazu Meana, Pablo Zunino, and Mariano Higes (2009) Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environmental Microbiology (in press).
Czekońska, K. 2000. The influence of Nosema apis on young honeybee queens and transmission of the disease from queens to workers. Apidologie 31(6): 701-706.
Chen YP, Evans JD, Murphy C, Gutell R, Zuker M, et al. (2009) Morphological, molecular, and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee, Apis mellifera. J Euk Microbiol 56: 142–147.
Fries, I., Forsgren, E. 2009. Nosema ceranae fungerar inte som Nosema
apis. Bitidningen 107, juni, 20-21.
Higes, Mariano , Raquel Martín-Hernández , Encarna Garrido-Bailón , Pilar García-Palencia , Aránzazu Meana (2007) Detection of infective Nosema ceranae (Microsporidia) spores in corbicular pollen of forager honeybees. J Invertebr Pathol. 2007 Jun 20; : 17651750
Higes M, Martin-Hernandez R, Botias C, Bailon EG, Gonzalez-Porto AV, et al. (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol 10: 2659–2669. tion and preventative treatment with fumagillin against Nosema ceranae. J Apicult Res 47: 84–86.
Kralj, J, S. Fuchs, J. Tautz (2006) Disease removal by altered flight behavior of forager honey bees (Apis mellifera) infested with Nosema apis. Proceedings of the Second European Conference of Apidology EurBee.
Lehnert, T, H Shimanuki, and D. Knox (1973) Transmission of nosema disease from infected workers of the honey bee, to queens in queen mailing cages. ABJ Nov 1973: 413-414.
Loskotova J., Peroutka M., Vesely V., Nosema disease of honeybee queens (Apis mellifica L.). Apidologie 11 (1980) 153–161.
Malonea, LA and HS Gatehouse (née Edmonds) (1998) Effects of Nosema apis infection on honey bee (Apis mellifera). J. Invertebrate Pathology 71(2): 169-174.
Martín-Hernández, Raquel, Aránzazu Meana, Lourdes Prieto, Amparo Martínez Salvador, Encarna Garrido-Bailón, and Mariano Higes (2007) The outcome of the colonization of Apis mellifera by Nosema ceranae. Applied and Environmental Microbiology http://aem.asm.org/cgi/content/abstract/AEM.00270-07v1
Martín-Hernández R, Meana A, García-Palencia P, Marín P, Botías C, Garrido-Bailón E, et al. (2009) Effect of temperature on the biotic potential of honeybee microsporidia. Applied and Environmental Microbiology. 75(8):2554-7.
Mayack, C., Naug, D.(2009) Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection, J. Invertebr. Pathol. 100:185-188.
Pajuelo, AG, C Torres and FJ Orantes Bermejo (2008) Colony losses: a double blind trial on the influence of supplementary protein nutrition and preventative treatment with fumagillin against Nosema ceranae. Journal of Apicultural Research 47(1): 84-86.
Plischuk, S, R Martín-Hernández, L Prieto, M Lucía, C Botías, A Meana, AH Abrahamovich, C Lange, and Mariano Higes (2009) South American native bumblebees (Hymenoptera: Apidae) infected by Nosema ceranae (Microsporidia), an emerging pathogen of honeybees (Apis mellifera). Environmental Microbiology Reports (2009) 1(2), 131–135.
Starks, P.T., C.A. Blackie , and T.D. Seeley. 2000. Fever in honey bee colonies. Naturwissenschaften. 87:229-231.
Webster, Thomas C, Etta M.Thacker, ,Kirk Pomper, Jeremiah Lowe, Greg Hunt (2004) Nosema apis infection in worker and queen Apis mellifera. Apidologie 35: 49–54.
Woyciechowski M., Kozlowski J. (1998). Division of labour by division of risk according to worker life expectancy in the honey bee (Apis mellifera L.). Apidologie 29:191-205.
Yafimenko, T, and L Bodnarchuk (1994) Some properties of host parasite interactions between honey bees from different generations and their microsporidial parasite, Nosema apis. 12th Congress of the International Union for the Study of Social Insects JUSSI Paris, Sorbonne, 21-27 August 1994. p. 348.
Youssef, N N, and D M Hammond (1971) The fine structure of the developmental stages of the microsporidian Nosema apis. Tissue and Cell 3:283-294.