The Learning Curve – Part 1: 2009 Progress Report
The Learning Curve—2009
Randy Oliver
Scientificbeekeeping.com
First Published in ABJ in April 2009
Physicist Neils Bohr once quipped, “An expert is a person who has made all the mistakes that can be made in a very narrow field.” This definition clearly excludes me from being any sort of expert, since I exuberantly continue to make new mistakes in my own beekeeping adventures. It appears unlikely that, hard as I try, I will ever make them all.
Years ago I was riding along to the almonds with beekeeper Gary McClaughry. He joked that he was thinking about writing a book entitled “My First Thousand Mistakes in Beekeeping.” He hasn’t written it yet, and perhaps some day I’ll steal the title from him (I’ve already made enough mistakes for a sequel, too!).
I often chuckle when a beekeeper, after reading my articles, approaches me as though I am some sort of great beekeeper who knows all the answers. In reality, that illusion is due to the fact that (1) he’s never seen the mess at my home yard, and (2) he simply doesn’t realize how many unanswerable questions there really are!
The learning curve in beekeeping goes something like this: One starts out the first season overwhelmed by all the information, and makes a slew of mistakes that year. But if he or she sticks with it and successfully gets some sort of honey crop the second or third year, he may well feel that he is close to the top of the learning curve of knowing all that there is to know about beekeeping. So if you want to talk to someone who can give you a definitive answer to any beekeeping question, ask a second- or third-year beekeeper. Because from that point on, you begin to realize how little you really know! Indeed, each year the learning curve ahead of you appears to loom larger. This is part of the wonderful challenge of beekeeping.
A couple of years ago I was chatting with Richard Adee (who can wear the title of “The World’s Largest Beekeeper”). Despite his vast knowledge from a life of beekeeping experience, he expressed his delight that beekeeping was a field of endeavor to which you could devote lifelong study, yet know that you would always have questions yet to answer. That sort of inquisitive and humble attitude is a common attribute of the best beekeepers!
Colonies sicken and die. Get over it!
I’ve taught beginning beekeepers for over twenty years. After seeing the guilt and embarrassment that some feel when their colonies die for lack of proper husbandry, I always include in my beginners classes a personal story from a gazillion years ago when I was a graduate student in fisheries biology. Part of our project involved the raising of thousands of salmon fingerlings in large tanks of recirculated water.
One morning, when I arrived at the rearing facility, my buddy Jim met me at the door, white faced and shaken. He explained that he had screwed up and closed the wrong valve the night before, and now several thousand fingerlings were floating belly up. Our major prof was due to arrive in a few minutes, and Jim didn’t know how he was going to explain that he had suffocated the fish, and ruined the whole experiment.
When the professor arrived, he calmly took the news in stride, then spoke a brief yet memorable sentence to my distraught friend, “Jim, you’re not considered to be a real fish culturist until you’ve killed your first million fish!” It took a few moments for the meaning to set in—the price for being able to advance up the learning curve is the experience gained by multiple failures, more so than from lucky success.
My point to new beekeepers is to not beat yourselves up over lost colonies (OK, there’s no excuse for letting them starve). Bees sicken and die, with or without your help, and especially since the arrival of the varroa mite. When you fall off the horse, dust yourself off, and get back on the horse. This is exactly what the Adee clan did last year after their devastating losses (as well as did the majority of other beekeepers who were decimated by CCD). Despite all the problems facing bees and beekeepers, I’m happy to report that the industry appears to be on the rebound.
Varroa management, revisited
A couple of years ago I began a series of articles on the management of the varroa mite! Since that time CCD reared its ugly head, we discovered that Nosema ceranae had snuck into the U.S., beekeepers learned to pronounce the word “imidacloprid,” and the feeding of pollen supplements became commonplace.
These past years have been a real learning curve for me, and for the industry as a whole. I am greatly indebted to all the researchers and beekeepers who have shared information and collaborated with me to try to make sense of what has been happening. I feel that it might be valuable to bring us all up to date at the start of this season (unless, that is, you started your season midwinter in the California almond orchards). Please allow me to review and update the topics I’ve covered, in roughly the same order that they were published in this Journal. Please note that this article is only going to be shorthand updates—you can read the original articles in the back issues, or at www.scientificbeekeeping.com.
Nutrition
I began my series with a discussion of the concept of Integrated Pest Management (IPM) for varroa. At the top of my list of cost-effective management techniques is making sure that your bees obtain adequate nutrition. Research by Dr. Frank Eischen confirmed that colonies winter better and stronger, and handle parasites better if they are well fed. Beekeepers everywhere are finding that protein supplementation during times of pollen dearth results in healthier colonies.
The health and strength of a colony is based largely upon two factors—constant replacement of older bees with fresh brood, and the length of foraging life of adult bees before they die. Both of these factors are largely dependent of the daily state of colony nutrition, especially with regard to protein, which is supplied by pollen. Furthermore, the wintering ability of the colony is directly proportional to the amount of protein available in late summer and fall.
Beekeepers can practice better husbandry by either moving bees to good late summer pasture (this generally is the most cost effective), or by feeding them pollen supplement. If colonies have plenty of stores, protein supplementation alone may be enough to allow them to continue rearing fresh brood. If not, feeding as little as ½ gallon of light syrup per week will help to maintain an active broodnest.
On the other hand, strong colonies can benefit from a lot of pollen supplement. My friend Keith Jarrett has shown me that it is cost effective to feed several pounds of supplement at a time, in order to avoid the labor required for weekly feedings. Keith is a first-rate, sharp-pencil, commercial pollinator, who produces huge wintering colonies by large-scale supplemental feeding.
I caught Dennis Lohman ( lohmanapiaries@frontiernet.net This e-mail address is being protected from spam bots, you need JavaScript enabled to view it ) assembling one of his mixers specifically designed for blending pollen supplement. Dennis runs bee operations in California and Mexico, produces honey in the Dakotas, pollinates and brokers bees, produces queens and packages, and designs and manufactures a line of stainless steel extracting equipment!
The race is clearly on for who can develop the best pollen supplement (no one has yet developed a pollen substitute). It needs to be palatable, cost effective, and supply the necessary nutrients for colony growth and disease resistance, as well as having no toxic effects (such as may be found with some soy, milk products, salts, or drugs). I hesitate to make any recommendations, since proprietary interests are at stake. I’ve posted two tested formulas to my website.
A pollen supplement feeding trial in one of my colonies. Not only must a supplement be palatable, but it must be nutritionally as complete as possible, and not contain ingredients that may be harmful to bee health. All photos by the author.
An even lower-tech colony nutrition solution is simply to place fewer hives in each yard to minimize competition for forage (this also helps to reduce parasite transfer). Several beekeepers, including myself, are having great success by cutting our traditional stocking rates in half. You may find that you have less problem with varroa in smaller yards.
Then there’s the HFCS/sucrose debate. Most trials find sucrose to be a better feed, especially for stimulation and buildup, and also for wintering. However, others report that bees treat high fructose corn syrup similarly to honey, and winter well on it, and that is has fewer granulation issues. Lots of California beekeepers are using HFCS/sucrose blends.
One aspect of HFCS is that the processing method may leave it quite acidic. Bees actually prefer acidic foods, but unfortunately the combination of sugar, water, acid, heat, plus a metal catalyst results in the rapid formation of bee-toxic hydroxymethylfurfural (HMF). That combination of factors exists in heated metal syrup tanks, or those left in the sun or a warm shed.
Beekeepers have had problems with batches of syrup gone bad. Definitely do not feed bees any syrup that has darkened, or has a bitter off flavor. However, a lab test is necessary to determine if the HMF has reached harmful levels. Dr. Jerry Bromenshenk found that beekeepers’ tanks could contain layers of syrup, as a result of dumping fresh syrup on top of old. Best practices would be to use plastic tanks, or if you use a metal tank, to clean it first, and use the syrup quickly.
For bee feeding in general, it is wise to use only high-quality fresh ingredients. Aged, off-spec, or floor sweepings may either have experienced nutritional degradation, or contain harmful contaminants. Ditto for syrup, including fermentation or dead bees in the feeders. A good rule of thumb is, If you wouldn’t be willing to eat or drink it yourself, you shouldn’t be feeding it to your bees.
Breeding
The ultimate resolution to our problem with varroa lies in bee breeding—anything else is simply a band-aid to get us by until we are all using “varroa-adapted” bees. Dr. Jose Villa (2008) reviews the recovery of feral bee populations after their initial decimation by varroa mites. It appears that if the unforgiving hand of natural selection is allowed to function, that feral populations come to terms with varroa in a time frame of 5-10 years. This has happened time and again throughout the world.
Unfortunately, what works in the relative low density of feral populations may not be enough to keep varroa at bay in colony-dense managed apiaries, yet. I will be covering this topic in detail in an upcoming series of articles. Although I am sanguine about the promise of breeding varroa-adapted bees, it is certainly frustrating to beekeepers that despite 20 years having passed since varroa’s arrival, most queen producers still aren’t able to offer such stocks.
However, two stocks that hold great promise are available: the VSH/SMR line maintained by Dr. Robert Danka of the ARS Baton Rouge Bee Lab, and the Russian bees developed by Dr. Tom Rinderer in conjunction with collaborators in the industry.
Dr. Tom Rinderer and Russian Bee breeder Carl Webb at Carl’s home apiary in Georgia. The members of the Russian Honeybee Breeders Association are very enthusiastic about their stock, which consists of 18 queen lines maintained to ensure genetic diversity. These bees often need no treatment for varroa or tracheal mites or hive beetles, and can produce honey crops comparable to those of Italian stocks.
The Russians are a good example of a mite-adapted bee (although not yet quite as good as the mite’s original host, Apis cerana). Rather than being a “one trick pony,” they exhibit multiple traits to fight the mite at every turn. This suite of special adaptations may be why Russian outbred crosses are not as mite resistant as purebred stock.
Although Russian bees have been successfully used in a number of geographical areas, it helps to be aware of their origins. They herald from the Primorsky region–an area with a cold winter, a cool, dry spring and summer, followed by a warmer, wet August and fall. They are adapted to winter very well with small clusters, and then to build up rapidly in response to pollen flows, and then shut down again to conserve stores.
The Russian Bee program has been selecting to improve the original Primorsky imports for better mite resistance, less propensity to swarm, and good honey production. The main fault that I hear about them is that they do not build up early enough for almond pollination (although recent research by Baton Rouge suggests that fall feeding and space restriction may alleviate this problem). They have been enthusiastically adopted by many small-scale non-migratory beekeepers.
I made the mistake of trying out Russian queens mated to Italian drones, and wasn’t impressed. I’ve since learned that the outcrossing of Russians doesn’t work well—it’s like trying to make a running truck by combining Chevy and Mercedes parts. After hearing the glowing praise given to Russians by many beekeepers that I’ve visited or spoken to, I’m going to give them another try this year, and see if I can tweak colony management (by feeding, etc.) to get them to build up early for almond pollination. They appear to be an excellent choice for nonmigratory beekeepers, especially in cold winter areas, or those with summer drought.
To me, the Russians, even though they are likely not the perfect bee for every operation, represent a model for a mite-adapted bee, and a foundation of potential breeding stock for continual improvement. My hat is off to Dr. Rinderer and the other dedicated researchers at Baton Rouge, as well as to Charlie Harper and the other cooperative breeders who have taken over the program!
The second available stock of interest is the VSH/SMR line. This trait, by which bees suppress mite reproduction by the removal of any reproducing mites from worker brood, is the most important trait in any varroa resistance breeding program. The stock is not touted as a production stock, but rather as a source for VSH alleles that can be bred into your existing stock. As far as I am concerned, any queen breeder should have plenty of VSH drone mothers in every mating yard in order to improve the mite resistance of his stock.
In my own 500-colony operation, after a brutal learning curve, I find that having the bees themselves do most of the hard work for mite control allows me to be a bit more complacent about varroa management, largely prevents colony losses due to varroa buildup, and saves me time and money (not to mention that I haven’t needed to use a synthetic miticide in over five years).
As I travel the country, in almost every area I find a few brave pioneers who are foregoing most or all treatments, and breeding their own mite-adapted stock. These (generally) small beekeepers often take serious losses, yet have the motivation and fortitude to continue, fueled by the progress they see, and the satisfaction gained from contributing to the long-term solution. I support them in their efforts wholeheartedly, and see their successes as harbingers of the future of beekeeping!
Mite monitoring and treatment thresholds
When I wrote about treatment thresholds two years ago, I was puzzled by the fact that some American researchers suggested that we could allow mite infestation levels in fall of up to or over 10% (10 mites per 100 bees), whereas the Europeans were adamant that infestations of over about 1% would be fatal. What I didn’t realize was that the Europeans were, as always, ahead of us in varroa experience.
The key to solving the puzzle is viruses. Bee viruses are normally not much of an issue without the presence of mites (varroa or tracheal) or Nosema (apis or ceranae). Dr. Mark Goodwin told me last week that colonies of bees in New Zealand can appear healthy with mite levels in the 20-30,000 range before viruses finally cause them to collapse! Dr. Mike Allsopp similarly documented mite levels of up to 50,000 when the mite initially arrived in South Africa. And anyone who witnessed the first years of varroa in the U.S. can probably remember the massive levels that mites could build to before the colony finally succumbed.
What changes is that the viruses, especially Deformed Wing Virus (DWV), apparently evolve to better take advantage of the mites as vectors (there is debate as to whether the viruses actually reproduce in the mite), or simply become more virulent. The result is that our mite thresholds now follow the European model, in which a 1000 mite total population is playing dangerously close to initiating a viral epidemic in the hive.
So let’s do the math (don’t you get tired of me saying that?). Let’s say that a moderately strong (15-frame strength) colony has 30,000 bees and a mite population of 900. About two-thirds of the mites will be hidden in the brood, leaving a third out on the bees. That would give you roughly 300 mites on the adult bees. Divide 300 mites by 30,000 bees, and that gives you a 1% infestation of the adult bees.
A 1% infestation of adult bees indicates that the colony is leaving the “safety zone.” Note that naturally mite-adapted races of bees seldom allow the mite level to exceed 2%. So I aim to intervene when mite levels look like they are going to exceed 1%, which in reality buys me a month of time before they actually double to 2%.

In any case, in the past few years, successful commercial beekeepers have learned to keep mite levels low all season. They now cringe at ether roll numbers that they used to ignore. I personally aim for no more than a 1-2% infestation by August 15. This is the critical date to get mite levels down, way down, so that the colony can rear a generation of winter bees that aren’t mite compromised or virus infected.
Since the mite population doubles roughly every month when the bees are rearing brood, you need to do the math before you put your honey supers on. Realize that mite levels may increase by a factor of four during a two-month honeyflow! I’ve found that it really helps to give my colonies a shot of Apiguard® thymol gel a couple of weeks prior to supering up for the main flow.
I regularly hear of beekeepers who put in a chemical treatment in September or October, that caused the stickyboards to “turn red with mites.” Sure, they can belatedly get mite levels down, but the damage has already been done to the wintering bees, viruses have likely already gone epidemic, and their colonies are nothing more than “dead bees walking.” It should be no surprise if those colonies don’t make it to spring.
Monitoring
When I receive calls from beekeepers whose bees are having problems, the first question that I ask them is, “What are your current mite levels?” If they answer that they are not sure, then I tell them, “Yes, you have a problem.”
Folks, varroa is still our number one problem. Not necessarily directly, but simply that an excessive varroa infection saps the strength of a colony, and gives viruses a foothold. What’s excessive? I can’t speak for all areas or operations, but as far as I can tell, once you get above about the 2% level, the mites are soon going to start taking a bite out colony production.
As a rule of thumb, mite levels typically follow the production of drones. Levels are at their lowest at the beginning of spring buildup, and will roughly double each month until they peak about the first of September. Use your own judgement and experience as to what levels you can feel comfortable with at which times of the year. During times of nutritional or disease stress, keep mite levels lower. If you use a mite-adapted stock, you may be able to give them a little more leeway at certain times of the year.
Successful beekeepers generally keep a close eye on that mite buildup, and take action before it gets out of hand. By doing so, they avoid a lot of costly surprises. By “action,” I mean either one of the biotechnical methods, or a synthetic chemical or “natural” treatment.
How best to monitor mite levels
Stickyboards counts of natural mite drop are OK if you get enough readings, but don’t put too much stock in their accuracy. Ditto with drone brood inspection. Better are “accelerated” mite drops, but their accuracy depends upon using an accelerant that works—the last chemical treatment that you used does not necessarily meet that criterion! A sugar dusting drop is my favorite—mechanical mode of action, quick, cheap, and easy.
Otherwise, I suggest a “jar” sample of 300 bees from the broodnest. Knock the mites off with ether, alcohol, detergent water, or powdered sugar. A caveat is that jar samples have the limitation that the sample of bees may not well represent the actual infection level of the entire colony. Always take multiple samples!
Monitor mite levels in early spring, again before supering, then at August 15th to see if you need to remove your supers and treat, and finally in late fall. Knowing the actual level of the pest is at the heart of any IPM program.
Biotechnical methods
Another aspect of IPM is management techniques. There is an old saw in the technology field: “Any new technology must be ten times as good as the thing it seeks to replace.” In beekeeping, we have a similar general resistance to change, but with the low margins of return for commercial operations, even small improvements tend to be eventually adopted in order to keep up with the competition.
In general, I see that commercial beekeepers rapidly adopt innovations that save money or time; whereas small beekeepers look for sustainability and lack of chemicals, and are willing to experiment further from the “norm.” In some aspects, the commercial guys are leading the industry. In others, the smalltimers are on the cutting edge.
Of the biotechnical methods, for the hobbyist/sideliner, we’ve got sugar dusting, small cell, and screened bottoms. I just covered sugar dusting last month, so I’ll skip directly small cell. A few studies comparing mite levels in small cell to “regular” (5.4mm) cell combs have been completed in the last year, but only one has been published (Taylor 2008). None found small cell to be of advantage in mite control, and at least one to the opposite! However, the few HoneySuperCell (HSC) colonies in my operation still chugged along just fine all season without any treatment. I don’t know what’s up.
My HSC experiment brings me to an example of my learning curve. Last spring, I moved the HSC test colonies to the almonds. I’d pollinated this orchard in previous years, but last year there was a change—a neighbor’s orchard had come into production, and my grower didn’t want the bees he had rented to fly to the neighbors. So he had me move some drops of 24 colonies from the edge of the orchard to the center. I thought that one of these drops would be a relatively isolated location for the test colonies. To my dismay, when I checked back a week later, the colonies appeared to have suddenly collapsed from CCD—the adult bees mostly gone, with just a handful of young bees and the queen trying to cover a large area of brood.
Forward to this year. I went back to the same orchard, and again set bees into one of the mid-orchard drops. I checked all the colonies individually for strength as they went in. Six days later I again checked all 152 colonies in that orchard one by one to make sure that they met my 11-frame contract topout. I needed to boost about a half dozen of them, but overall they had continued to grow during the six-day interim.
However, when I got to the 24-colony mid orchard drop, I was dumbfounded! All the colonies had again collapsed (no HSC in the group this time) with the same symptoms. I stood there thinking about it until my brain hurt. Then it struck me—this was a densely-planted six-year-old, mechanically-pruned orchard. Normally we place mid orchard drops where there is a tree missing, which creates a hole in the canopy that returning foragers can orient to. However, in this particular instance, the trees were absolutely uniform, and from a bees-eye view, completely lacking landmarks, other than edge details.
In this situation, all that the returning bees could see was a vast, uniform sea of gray, leafless, mechanically-pruned branch tips of uniform color, height, and density (my natural-colored hives unfortunately blended into the shades of the soil). The colonies were not sick—the foragers had apparently simply drifted to drops of hives at the row ends, thereby leaving the colonies depopulated. I’ve been pollinating almonds for over 25 years, yet this was a first (and expensive) lesson to me–one more tiny step up the learning curve!
Screened bottoms—the jury’s still out. They may provide some small degree of mite control, and are really handy for getting stickyboard counts, or for sugar dusting. They are great for summer ventilation, and don’t appear to be a problem in winter provided that you don’t have strong winds blowing under the hives, or if you slide in a solid closure sheet (an easy way to instantly change from screened- to solid bottom). They can be a problem when moving pallets with a forklift if the bees cluster under them.
This brings us to the most practical biotechnical method, with three variations on the theme:
Breaking the brood cycle
As I question successful commercial beekeepers on aspects of their management of varroa, one common practice stands out: the breaking of the brood cycle to interrupt mite reproduction.
The most common method of breaking the brood cycle is by making splits, or by shaking (or purchasing) packages. If the packages are relatively mite free, the new colony may well go a year before mites (and viruses) build to harmful levels. I spoke with one large Southern California outfit that shakes “packages” to make up new colonies in their own operation on a regular basis. They are able to get by with only one mite treatment a year! Others buy packages in the spring, then shake their bees out in the fall for sale to almond pollinators, and start fresh again next season.
Making up nucs does not break the varroa cycle as well as shaking broodless splits, but it can be improved upon by making them up with ripe queen cells, and then giving them some sort of varroa treatment just as the new queen’s first brood reaches the capping stage–you only have about a one-day window during which there is no sealed brood for the mites to hide in.
Kirk Webster in Vermont, checking 4-way nucs prior to winter. These queens, mated in the current season, will become his production queens next spring, after proving themselves over the winter.
A number of northern beekeepers are having success with making up weak nucs (1-2 frames of bees) during summer, and letting them build to wintering size, to provide the production colonies for the next year. One advantage of this technique is that each queen gets a chance to prove herself prior to supering her colony up for honey production. It also allows those beekeepers to raise their own queens for the next season.
A close up of one of Kirk’s 4-way nucs. Each feeder has separate entrances left and right. During winter, bees cluster in the empty feeders, and all four small colonies share their heat through the thin pressboard dividers.
There are other ways to break the brood cycle. Some hobbyists simply cage the queen for a couple of weeks. Or they can do it more naturally by using mite-adapted bees such as the Primorsky Russians—they shut down broodrearing at the first sign of pollen dearth, and scour out the then-exposed phoretic (hitchhiking) mites by vigorous grooming. This process sets the mite level back at least twice a year.
Drone brood trapping
Another method of breaking the brood cycle is by periodically removing the drone brood, in which most mite reproduction takes place. This is a cost-effective method for small- or (perhaps) large-scale beekeepers, especially if a cut-out (as opposed to freeze-kill) varroa trap frame is used (Oliver 2007). The questions for each individual operation are what times of year to do it, and how many times are optimal. I find that in my operation there is little benefit to cutting out the first round of drone brood, since my early spring mite levels are so low, following winter oxalic dribble. It may be best to check the drone frames with a cappings fork in order to determine when they contain enough mites that removal would be of benefit.
A further question is to determine just how many drone brood removals at appropriate times will be enough. In my own operation, I have found colonies in which I inadvertently left drone frames in all summer (yes, I screw up regularly). Some of those colonies still maintained low mite levels! Perhaps a couple of removals in mid spring would be adequate to set the mite population back—this would also avoid selecting for varroa specializing on worker brood (a potential problem noted by Dr. Roger Hoopingarner). We need more research to fine-tune the use of drone brood trap frames, especially to see if they are cost-effective in a commercial operation (I suspect that they would be).
Scorecard
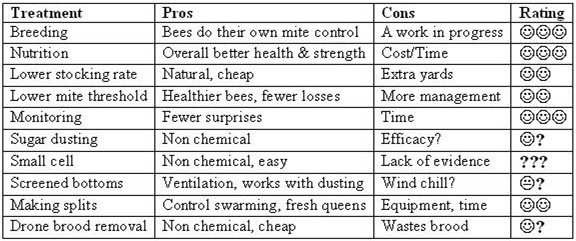
Next month
A review of the mite “treatments.”
References
Oliver, R (2007) Tactics: Biotechnical Methods (II). ABJ 147(5): 399-406.
Taylor, MA, RM Goodwin, HM McBrydie and HM Cox. (2008) The effect of honey bee worker brood cell size on Varroa destructor infestation and reproduction. J. Apic Research 47(4): 239-242.
Villa, JD, D Bustamante, JP Dunkley, LA Escobar (2008) Changes in Honey Bee (Hymenoptera: Apidae) Colony Swarming and Survival Pre- and Postarrival of Varroa destructor (Mesostigmata: Varroidae) in Louisiana. Annals of the Entomological Society of America 101(5): 867-871(5)








