Does The Crushing of Bees Affect Colony Health?
Beekeeper-Supported Research
Randy Oliver
ScientificBeekeeping.com
One concern that I have with the feeding of pollen sub is whether inadvertently crushing bees when you place the patties could lead to transmission of pathogens within the hive.
Introduction
There are a number of ways to feed supplementary protein to a colony—in patty form under the lid or between the brood chambers, in liquid form, or in dry form outside the hive. Each has its advantages and disadvantages (which I continue to investigate). To date, I’ve gotten best results by cracking open the brood chambers and placing patties on the top bars, where depending upon patty thickness and the amount of burr comb on the wood, they squish to fit.
A problem arises when one is feeding hundreds of hives a day, especially in cool weather–what to do about the bees clustered on the top and bottom bars? It can take a while to get the bees to move out of harm’s way, and under the pressure of time, one may be tempted to just go ahead and squash a few bees (Fig. 1).

Figure 1. I raised this patty back up after placing it on top of the bee-covered top bars, to show the ugly aftermath. It would have been even worse had I then lowered the upper box. My question is, to what degree such inadvertent crushing of bees affect colony health?
We all love our bees, and most beekeepers go out of our way to avoid killing any, not only out of respect for their lives, but also because the release of alarm pheromone may trigger increased stinging. But to the time-conscious beekeeper desperate to put patties into a thousand hives during rainy weather there is always the temptation to just throw them in and try to ignore the gruesome sound of bees being crushed as you close the hive.
A couple of years ago, in preparing a group of 48 healthy hives for a trial, I wanted to equalize the presence of inapparent pathogens throughout the group. So I created an inoculum by collecting about fifty apparently-healthy bees from the entrance of each of the hives, combining them all, homogenizing them in a blender, filtering the homogenate, mixing it with syrup, and then dribbling a portion back over the cluster in each of the 48 hives.
I didn’t expect to see any particular effect from the inoculation, since we had chosen only thriving, building colonies for the experiment (a test of various essential oil products, as yet unpublished). To our surprise, despite there being a decent nectar and pollen flow over the course of the next two months (during which we also fed plenty of syrup and pollen sub), the buildup of most of the colonies was immediately arrested (whereas that of those in nearby yards continued on normally). This so impressed us that we have since gone out of our way to avoid crushing bees in the hive.
Background
We are not the first to suspect a negative effect upon colony health from the inadvertent crushing of bees during normal hive manipulations. Keep in mind that prior to the cuticle-penetrating parasitic mites, pretty much the only way for a pathogen to gain entrance to a bee’s body was through the mouth. Bees thus evolved defense mechanisms that act as barriers to infection via the gut, and it is indeed difficult to infect adult bees with most pathogens in this manner. The most serious in-hive transmission of pathogens occurs when bees suffering from dysentery are unable to leave the hive for cleansing flights, and defecate on the combs. Since cleaner bees don’t have access to sponges and disinfectant, their only option for cleaning up the mess is to lick it up. Yuck.
Once licked up, infective spores are swept from the crop by the filtering mechanism of the proventriculus (to avoid contamination of the honey) and passed in a bolus into the gut [1], where they are contained by the peritrophic membrane, in which the bee’s immune mechanisms work to either prevent their germination or to destroy them. Unfortunately, there may be so many spores or virions in the feces that the immune system is overwhelmed, and the cleaner bees get sick. This is why dysentery is often associated with diseased colonies, whether or not the dysentery is caused by the parasite or not (I’ve seen scant evidence that nosema ever causes dysentery).
Humans, however, have added another avenue of transmission of pathogens—by the inadvertent crushing of bees during hive manipulations. As Dr. Ingemar Fries points out [2]:
The liquid remains of crushed bees are readily ingested by other bees [Fig. 2].

Figure 2. Immediately after a bee is crushed on the top bar, other bees clamber over it and rapidly lick up all its fluids. The inadvertent crushing of bees during hive manipulations is analogous to creating dysentery in the hive, and can facilitate the transmission of pathogens.
And although it seems like common sense, bee pathologists have long cautioned beekeepers to avoid crushing bees, since there is abundant evidence that ingested fluids can transmit bacterial septicaemia [3], Chronic Paralysis Virus [4], amoeba and gregarines, IAPV [5], and especially the nosemas [6].
A recurring theme of researchers dealing with nosema has long been for beekeepers to be cautious during hive manipulations to avoid any crushing of bees [7]. However, there appears to be scant actual field research to back up this recommendation. The few references that I could find were associative—colonies in Australia that were manipulated during the winter (there are honey flows during the Aussie winter) tended to have more problems with nosema. These studies were reviewed by Hornitzky [8], who noted that in the (apparently unpublished) field studies:
There was a clear association between low [nosema spore] counts and no manipulation of hives in the three months prior to moving bees to the almonds. The high counts were usually associated with hive manipulation.
More recently a trial was carried out to maximise bee populations by using a range of bee [protein] supplements. A surprising finding was that any benefit from the various supplements provided to the colonies was overridden by N. apis infection which was most likely to have been exacerbated in the test hives by manipulation associated with supplementary feeding. The control hives (not supplementary fed) performed best.
Practical application: Holy Cow! Could it be that careless tossing in of pollen sub patties could negate the beneficial effects by exacerbating the transmission of nosema (and perhaps other pathogens)?
Since I couldn’t find a single controlled study in which the effects of the crushing of bees in the hive was clearly determined, I decided to use some of your donations to run a trial.
Materials and Methods
Preparation
We ran the trial in the northern Sierra Foothills at about 2300 ft elevation, in a historically good apiary site (Fig. 3), which we stocked on April 1, 2013 [9] with 96 two-way 4-frame queenless nucs, into which we then placed ripe queen cells of mongrel Italian stock.

Figure 3. The trial yard, with an adjacent creek sporting blackberry and alders, surrounded by pasture and woodlands. Although we were in a drought year, the colonies fared well in this location.
On April 20 we checked for queenrightness, and combined the queenless nucs with queenright to build them to 5 frames. On May 14 we worked the colonies into 49 singles filled with drawn combs. At this time there was a light nectar flow on.
By May 24 the blackberry was in bloom, although the nectar flow was light due to drought. The colonies were mostly of 6-10 frame strength, full of sealed brood and looking good. We picked the best 36 colonies for the trial (removing the rest from the yard).
May 27 (Time point 0): Cool day, very light rain, few bees foraging. Numbered, graded and weighed all hives. Although we had attempted to equalize by looking from the top three days previously, there was greater variation when graded by tipping up the hives and viewing from the bottom. We used a random number generator to assign treatment to half the hives (n = 18 ea, Test and Control).
Treatment: smoked the entrance of each of test colony, lifted the lid, waited for bees to cover top bars, rolled a rubber wheel over the top bars until I had crushed about 30 bees (eyeball estimate). Crushed hard enough to see guts extrude, trying to mimic bees being accidentally crushed during hive manipulations (Figs. 4 & 5). For the control group, we smoked each hive without opening it, so as to avoid crushing any bees. We repeated these treatments monthly for the duration of the trial.
We then added a deep super of foundation to each hive. Gave each hive ½ gal of 1:1 sucrose syrup. Blackberry bloom in immediate vicinity at about 50% stage.

Figure 4. Using a rubber wheel to crush about 30 bees on the top bars. We alternated two wheels, wiping each with 70% isopropanol between hives to avoid hive-to-hive cross contamination. Surprisingly, I found that I did not need to wear gloves to do this.
![Figure 5. The aftermath was pretty ugly. Within minutes, the rest of the bees will suck these crushed bees dry. We repeated this treatment once every month for 8 consecutive months. I arbitrarily chose 30 bees since this is the amount of bees that it seems that a careless beekeeper kills every time he or she opens a hive [10].](https://scientificbeekeeping.com/scibeeimages/fig-057-600x450.png)
Figure 5. The aftermath was pretty ugly. Within minutes, the rest of the bees will suck these crushed bees dry. We repeated this treatment once every month for 8 consecutive months. I arbitrarily chose 30 bees since this is the amount of bees that it seems that a careless beekeeper kills every time he or she opens a hive [10].
After a few moments, bees would then start to well up and approach the crushed bees, and immediately begin licking up their body fluids. At this time, if I attempted a second round of crushing, the colony would initiate a strong defensive response, stinging enthusiastically. We were never able to determine whether the initial withdrawal of the bees was due to my motion, the odor of the crushed bees, or the vibrations of the wheel. All the above behaviors were consistent from colony to colony, and over the entire course of the trial. I rarely needed to put on a glove or veil.
From May 31 through July we fed another gallon of syrup. The weather turned hot, and the blackberry completed its bloom. Most colonies drew some foundation. On July 11 we treated with ~25g of Apiguard between the brood boxes to control varroa.
July 26 (Time point 1): Hot weather. Graded, weighed, and crushed bees. Due to heat, clusters were broken and bees moving quickly. Due to clusters being spread out, grading was rather subjective, but all by me, blinded as to treatment.
During August through early November, we treated with 2 MAQS formic acid strips, fed another gallon of syrup plus 7 lbs of pollen sub patties, and a Sept 25 treatment with ~25 g of Apiguard.
November 13 (Time point 2): Weighed and graded all colonies (Fig. 6).

Figure 6. Eric and I are using a digital crane scale hooked to a boom hive loader to weigh the hives. Conditions were dry due to drought. The green vegetation is on the banks of a perennial stream that nearly dried up.
From late November through January we continued to feed (another half gallon of syrup and another pound of pollen sub), and treat for mites (oxalic dribble on Dec 13). We noted excessive amounts of dead bees in front of three hives (two controls, one test hive). One colony (control) removed as queenless.
January 26: Final grading: Graded all colonies for strength (by me). Two Control Group colonies had crashed to tiny clusters, both were still queenright; one Test colony had gone from 13 frames in July to dead.
Results
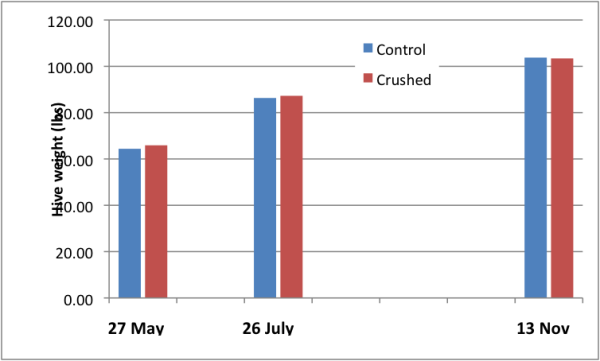
There was no difference in weight gain between the Test and Control groups (Fig. 7).
There was no difference in colony strengths between the groups at the July 26 grading, but a significant difference (Student’s t, p = 0.02) by the end of January (Fig. 8).
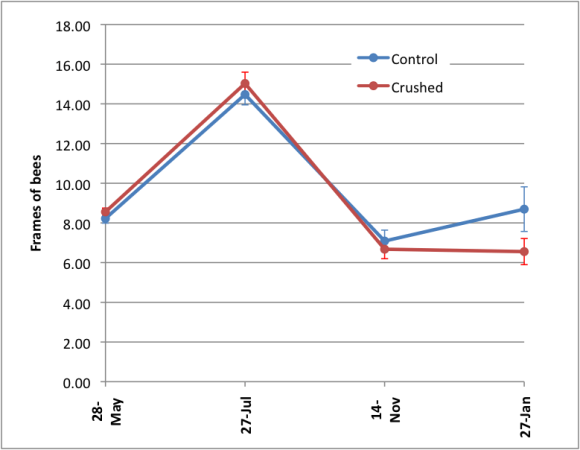
Figure 8. Comparison of colony strengths over the course of the trial. Note how the crushing of bees did not appear to negatively affect the colonies until winter.
The most telling result was the distribution of ending strengths (Fig. 9).
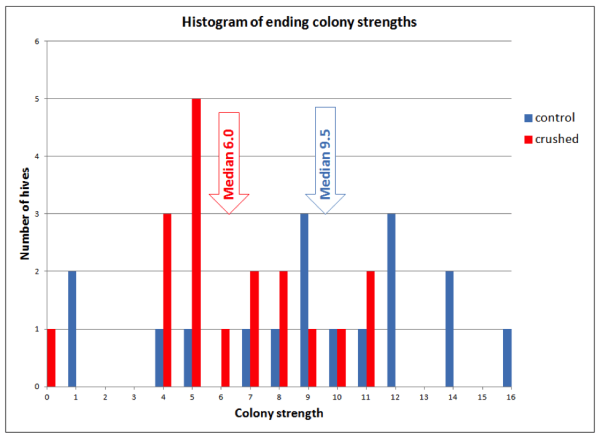
Figure 9. By the end of the trial the control colonies were consistently stronger than those which had bees crushed each month. Note that the 6 strongest colonies were all in the Control group, and 9 of 13 of the weakest colonies were in the Crushed Bee group.
Pathogen Analysis
I hadn’t planned on going to the expense of pathogen analysis, but the results were so striking that at the last grading I arbitrarily took bee samples from 8 of the weakest and 4 of the strongest hives, regardless of group (8 Controls, 4 Tests). In the individual gut squashes of 10-bee samples, only 5 samples were positive for nosema—all from weak hives, regardless of group (although nosema may have been somewhat more prevalent in the crushed bee group). In only one of the samples was more than 1 bee out of 10 infected, strongly suggesting that nosema was not the reason for the differences in colony strengths between the groups.
For virus analysis I chose to use Dave Wick’s IVDS method, since it quantifies the number of virions regardless of whether they are identified bee pathogens. There did not appear to be any consistent differences in either virus diversity or total virion count between the samples, again with perhaps slightly higher virion counts in weak colonies regardless of group.
Discussion
The lack of noticeable effect from crushing bees in the hive during summer is not surprising if you look at the colony demographics chart in my other article in this issue. The turnover of bees is so rapid during the summer that sick bees can remove themselves from the hive before they transmit pathogens.
The chart also shows why crushing bees during the winter would likely have a negative effect on the aging population of “winter bees.”
Surprisingly, neither nosema nor viruses appeared to be associated with the poorer performance of the crushed bee colonies. I have no idea which pathogens were involved, or even if it was indeed due to increased pathogen transmission. But it was pretty clear that you don’t want to crush bees in the hive during winter.
Conclusions:
- Crushing of bees didn’t seem to make a difference during summer.
- But did appear to negatively affect colonies during winter.
- Arbitrary sampling of the weakest and strongest colonies at final grading didn’t show any obvious differences in viruses or nosema
- There was little nosema overall, but slightly more in weak hives.
- We use gentle smoke and a bee brush (with the bristle tips leading) to clear the top and bottom bars of bees before we place any patty during the winter.
Something Else to Worry About?
I look at a fair amount of bee gut samples under the ‘scope. In early January I received a call from a desperate California commercial beekeeper whose colonies weren’t building, and were exhibiting an excessive amount of dark defecation along with some dysentery. He’d been dumping in fumagillin to no avail. I offered to take a look at some samples, so he sent me seven, mostly of bees, but some dysentery alone.
I quickly determined that nosema wasn’t his problem, since all of his samples save one were completely free of spores (and even that at a low level).
Practical application: don’t waste your money on fumagillin if you haven’t determined by microscopy that your bees are actually infected. Fumagillin is an immune suppressor, and you may not only be throwing money down the drain, but actually harming your colonies.
What I did notice in all his samples, however, is a critter that I hadn’t previously seen (Figs. 10 & 11)—an amoeba-like microorganism slightly larger than a nosema cyst. His bees’ guts were full of ‘em. I suspect that they were the cause of the dysentery, since other nearby apiaries foraging on the same pollen weren’t having problems. So far, experts that I’ve consulted have been unable to identify it.
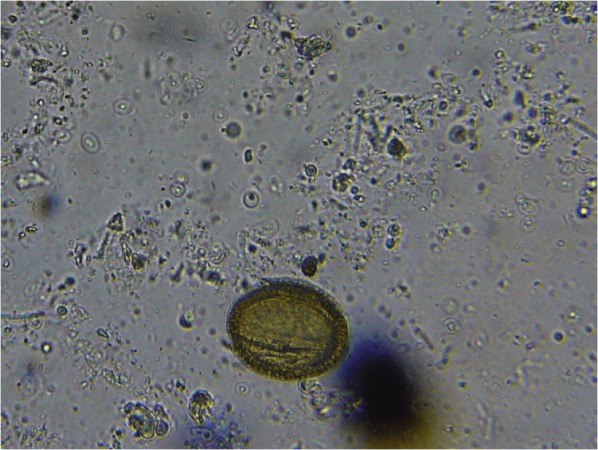
Figure 10. I included a pollen grain for scale in this photo, taken at 1000x with oil immersion. The mystery critters are the smaller oval outlines with the glowing or dark centers, which appear to be some sort of microorganism.
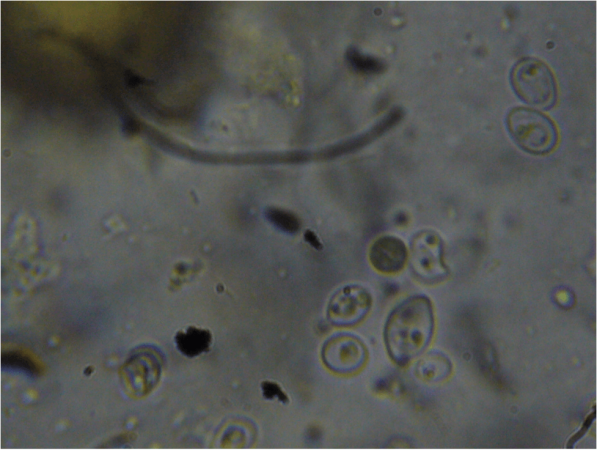
Figure 11. At higher magnification, one can clearly see what appear to be organelles in the unidentified organism.
I can’t yet say whether this organism is a pathogen that is causing disease—but I sure I hope that we don’t all catch it in almonds…
Update: I’m now guessing that this was a yeast infection.
Acknowledgements
This project was funded by beekeeper donations, with special thanks to the North Dakota Department of Agriculture. Thanks to my nosema technician Leslie Gault, and to Dave Wick for virus detection. To my sons Eric and Ian for their meticulous field work. And to Peter Borst for his research assistance.
Notes and Citations
[1] Peng, Y-S and JM Marston (1986) Filtering mechanism of the honey bee proventriculus. Physiological Entomology 11(4): 433–439.
[2] Fries, I (1993) Nosema apis – a parasite in the honey bee colony. Bee World 74(1): 5-19.
[3] Langridge, DF (1963) Septicaemia disease of the honeybee in Victoria. Australian Journal of Experimental Agriculture and Animal Husbandry 3(10) 225 – 227.
[4] Rinderer, TE (1975) The etiological agent of hairless-black syndrome of the adult honey bee, Apis mellifera L., and certain factors influencing its infectivity. PhD Thesis, Ohio State University.
[5] My own inoculation, while collaborating with Beeologics, of heathy hives with purified IAPV in syrup.
[6] Fries, I. (1993) op. cit.
[7] Fries, I & S Camazine (2001) Implications of horizontal and vertical pathogen transmission for honey bee epidemiology Apidologie 32: 199–214.
[8] Hornitzky, M (2008) Nosema Disease: Literature review and three year survey of beekeepers, Part 2. RIRDC Publication No 08/006
[9] The initiation of this study on April Fool’s Day was purely coincidental.
[10] Just kidding. But it’s really easy to crush 30 bees when casually tossing in a pollen patty or replacing a box when there is bridge comb built between the top and bottom bars.