Sick Bees – Part 18F5: Colony Collapse Revisited – Synthetic Pesticides
Sick Bees Part 18f5: Colony Collapse Revisited
Synthetic Pesticides
First published in ABJ May 2013
Randy Oliver
ScientificBeekeeping.com
Interactions Between Synthetic and Natural Toxins
Toxicological Eras in Honey Bee Evolution
Addendum: The Beekeepers’ Responsibility
Randy Oliver
ScientificBeekeeping.com
OK, I hope that since explaining that bees have always had to deal with natural plant toxins, and more recently, with human pollution, that I can finally move on to attempting to answer the original question, “To what extent are manmade pesticides related to colony morbidity, mortality, or sudden collapse?”
Synthetic Pesticides
Not being a toxicologist, I had always assumed that synthetic pesticides were chemically and biologically in a different class than the natural allelochemicals found in nature. How wrong I was!
Synthetic insecticides are essentially nothing more than chemically “tweaked” forms of natural substances, generally modified to make them cheaper, more effective, more or less stable, less toxic to humans, more targeted toward specific pests, and recently, more environmentally friendly. For a good summary of this subject, read John Tierney’s “Synthetic v. Natural Pesticides” [1].
Preadaptation
There is nothing new about honey bee exposure to pesticides—bees had by necessity been forced to develop detoxification mechanisms for these classes of chemicals long before humans invented modern pesticides! Prior to that (and still today), bees were exposed to naturally-occurring organochlorides from many natural sources [2], organophosphates produced by cyanobacteria in surface waters [3], carbamates as a natural fermentation byproduct of beebread [4], nicotine (as in neonicotinoids) in a number of plant species [5], pyrethrins (derived from chrysanthemums) [6], insect growth regulators (self-produced hormones), and a host of alkaloids and other toxins in pollen and nectar.
A term used by toxicologists is preadaptation. Honey bees are, by necessity, preadapted to deal with the major classes of synthetic pesticides; the toxicology and metabolism of synthetic insecticides is no different than that for natural toxins (although the synthetics may have a greater degree of toxicity). And despite the widely-cited paucity of detoxification genes in the honey bee genome, Hardstone [7] determined that compared to insects in general, honey bees are not particularly sensitive to insecticides overall, nor even to specific classes of insecticides!
If it hasn’t already occurred to you, think on this: there are any number of nectar/honeydew sources that bees concentrate into honey that may be acutely toxic to humans (rhododendron, mountain laurel, tutu, etc.), yet does not appear to affect the bees to any great extent. The toxins of those named plants (grayanotoxin and tutin) are poisonous to insects, yet bees are able to detoxify them better than humans can!
Cresswell [8] notes that some bees may be better preadapted to toxins than others. Remember that I mentioned earlier that tropical nectars tend to contain more alkaloids? Well, honey bees evolved in the tropics, and are apparently well preadapted to metabolize alkaloids, whereas bumblebees evolved in temperate regions in which there were fewer natural alkaloids in the nectars. It’s possible that honeybees may be better preadapted to detoxify alkaloids (such as neonicotinoids) than are bumblebees.
Have you noticed yet that this is a complex subject? And that is one reason why I feel that the single-minded focus by some folk on any one particular class of insecticides may be misguided. Lest I sound critical of my fellow environmentalists, I suspect that many remain under the misassumption that all pesticides bioaccumulate or biomagnify as do the “Persistent Organic Pollutants” (DDT, chlordane, PCB’s, etc) and heavy metals (mercury, lead). Gold [9] explains:
DDT is unusual with respect to bioconcentration, and because of its chlorine substituents it takes longer to degrade in nature than most chemicals; however, these are properties of relatively few synthetic chemicals. In addition, many thousands of chlorinated chemicals are produced in nature… Natural pesticides can also bioconcentrate if they are fat soluble. Potatoes, for example, naturally contain the fat-soluble neurotoxins solanine and chaconine, which can be detected in the bloodstream of all potato eaters.
Oh no—not only do French fries contain toxic acrylamide, but also additional neurotoxins that bioaccumulate in my body fat!
Reality check: our diet, as well as that of the bees, is chock full of natural plant toxins (many of which have been only recently been introduced into the human diet). The bee immune* system does not differentiate between natural toxins, environmental pollutants, or synthetic pesticides. They must all be taken into consideration when we discuss “chemicals” and bees. Rather than focusing on this pesticide or that, what we beekeepers should be assessing is the total toxin load to which colonies in any particular setting are exposed.
Update 4/27/2013: It’s been pointed out to me that I’ve used the term “immune” too loosely. I should have used the term “detoxification.”
Interactions Between Synthetic and Natural Toxins
Bees in agricultural landscapes, as well as in urban and suburban areas, are exposed to a wide variety of manmade toxicants above the background level of natural toxins. Surprisingly, previous exposure to plant allelochemicals may help them to deal with manmade toxicants!
Després [10] found that eating certain natural toxins in a plant may then make an insect more resistant to certain synthetic pesticides. Armyworms fed cowpeas became more tolerant to organophosphates. And those fed xanthotoxin from corn displayed higher tolerance to a pyrethroid insecticide—and appeared to be able to pass that immunity on to their offspring! Don’t you just love this stuff!
Biological note: we’ve barely investigated to what degree the exposure of the previous generation of bees to alleleochemicals or pesticides results in trans-generational epigenetic effects.
On the other hand, Després also found that:
By contrast, exposure to particular plant chemicals can repress the expression of detoxification enzymes involved in insecticide resistance…Finally, it cannot be excluded that an enzyme conferring resistance to a phytotoxin can enhance the toxicity of an insecticide and vice versa. The striking complexity of the repression–induction patterns and substrate specificities of detoxification enzymes has so far represented a major difficulty in the understanding of cross-resistance mechanisms.
“Striking complexity”—well put! Even the type of honey that bees are eating enters into the picture. A study by Mao [11] found that allelochemicals in honey may affect their ability to metabolize pesticides. The researchers also speculate that the practice of wintering bees on sugar syrup may compromise their ability to process environmental toxins!
I hope you are starting to understand why I couldn’t just jump into answering the question as to whether pesticides cause CCD! There are a great many contributory variables when we start looking at toxicity, and we just don’t yet know that much about a lot of them! But there is one thing that we do know—that there was a major change in honey bee exposure to toxicants starting (in this country) in the 1990’s.
Toxicological Eras in Honey Bee Evolution
Let’s imagine what honey bee exposure to toxins would look like from an evolutionary perspective (Table 1):
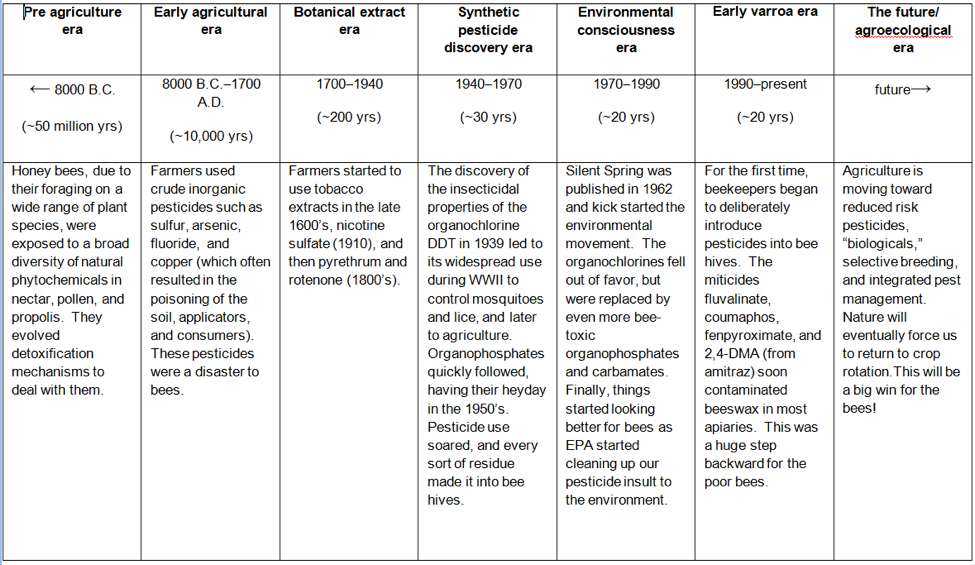
Table 1. Note that honey bees had tens of millions of years to evolve detoxification mechanisms for plant allelochemicals and other natural toxins. They’ve had only an evolutionary eyeblink of about 70 years of dealing with added synthetic pesticides. But the biggest change has been in the past 20 years, as beekeepers inadvertently contaminated beeswax combs with miticide residues in their attempts to control parasitic mites.
The “Varroa Era”
After 50 million years of evolving effective detoxification mechanisms against natural chemicals, mankind has only recently (on the evolutionary scale) challenged the bee with additional doses of synthetic toxicants. But those exposures were normally inadvertent and only intermittent, allowing the colonies to recover.
The invasion of the parasitic mites ushered in a new era of toxicant exposure [12]. Beekeepers were largely unaware that the synthetic miticides that appeared to be their salvation would wind up contaminating their combs with unimaginably persistent residues. I’ve seen the analyses of many beekeepers’ combs. The miticide residues reflect a history of any product that they’d put into their hives in the past decade!
And although there is a great hue and cry about the neonicotinoids of late, Frazier [13] (a must read) points out:
Indeed, if a relative hazard to honey bees is calculated as the product of mean residue times frequency detected divided by the LD50, the hazard due to pyrethroid residues is three-times greater than that of neonicotinoids detected in pollen samples.
The main pyrethroid in hives worldwide is tau-fluvalinate (Apistan, Mavrik), which accumulates in the combs, along with several other agricultural pyrethroids of additive toxicity. To make matters worse, the other common miticide residue, coumaphos (Checkmite+), then exhibits synergistic toxicity with those pyrethroids [14]. Surprisingly, both of these miticides are still sold in bee supply catalogs!
I’ve written at length about the synthetic miticides [15]. It’s hard to find combs today that aren’t contaminated with fluvalinate and coumaphos. Could this be a problem?
There was a significant reduction in adult bee longevity following exposure to 100 ppb of coumaphos in wax during the larval and pupal stages in worker honey bees. A 4-day reduction in summer bee lifespan was observed equaling 16 percent of the total lifespan of summer bees. Reduced adult longevity could impact honey production and or overwintering ability [16].
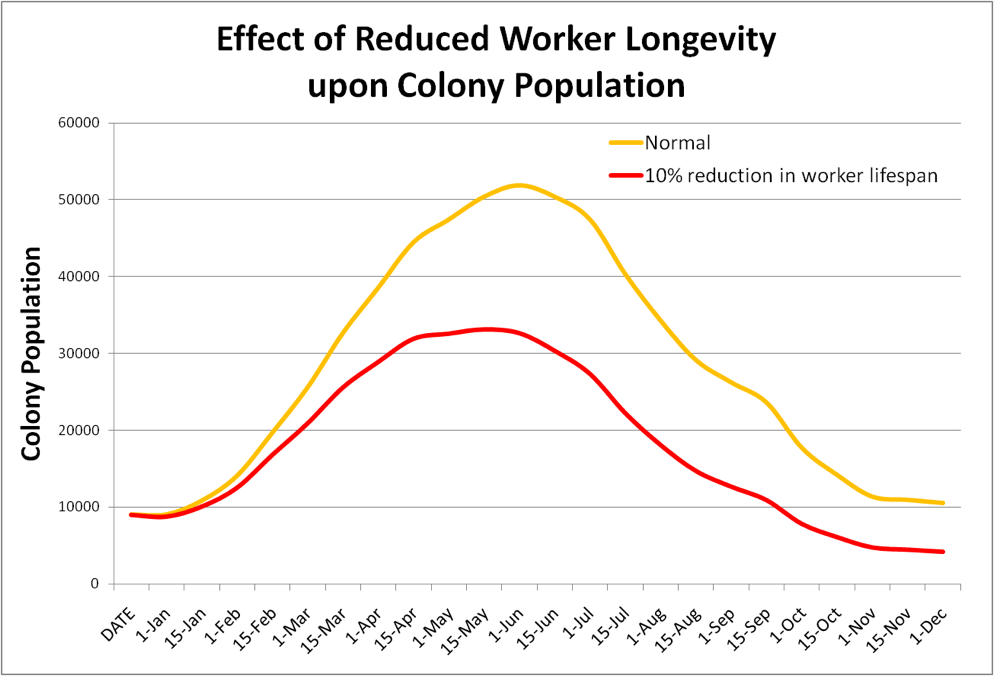
Their conclusion is an understatement! I’ve plugged a 10% reduction in worker longevity into my bee population model—the result is striking (Fig. 1)!
Figure 1. In this crude example, I reduced worker lifespan by 10% on the red plot, assuming a normal mean longevity in Feb-Oct of 35 days; and in Nov-Jan of 60 days. It is easy to see that even a slight reduction in worker longevity dramatically affects colony buildup and wintering ability!
Practical application: Miticide residues (or environmental pollutants) in the combs can affect both larval survival and worker longevity. It doesn’t take much of a reduction in either to demonstrably affect colony buildup or winter survival! And this is before adding any agricultural pesticides!
Some miticide residues don’t go away—they leach out of the wax and into the beebread for years! Add their negative impact on brood and adult bee survival to that of other environmental toxins and plant allelechemicals, and today’s colonies may already be in toxicological trouble even before they are exposed to any additional pesticides.
Practical application: honey bees have suffered from a “triple whammy” due to the recent invasion of the parasitic mites:
- 1. The mites directly impact the health of bees by sucking their blood, and by suppressing their immune systems.
- 2. Varroa changed the entire virus dynamics in the bee population, especially by acting as a novel and effective vector of some viruses.
- 3. Bees in many operations now must deal with an elevated background exposure to the toxic beekeeper-applied miticides, 24/7, 365 days a year.
- 4. This background exposure may increase larval mortality, adult bee longevity, and bee immunological resistance to mites, viruses, and nosema [17].
Since the invention of fat-soluble synthetic insecticides, for the first time in their evolutionary history, bees must deal with constant exposure to toxins not only from outside, but also leaching back out of the combs! Johnson, et al [18] found:
Overall, pyrethroids and organophosphates dominated total wax and bee residues followed by fungicides, systemics, carbamates, and herbicides, whereas fungicides prevailed in pollen followed by organophosphates, systemics, pyrethroids, carbamates, and herbicides. Externally-derived, highly toxic pyrethroids were the most frequent and dominant class of insecticides samples… Beekeepers searching for the primary source of pesticides contaminating bee hives need only to look in a mirror.
Such constant exposure to these elevated levels of toxicants keeps the detoxification crew bailing all the time in order to keep the bee boat afloat! Boncristiani [19] suggests that:
Since honey bees are constantly exposed to different compounds in nature, the activation of detoxification pathways probably do not necessarily represent negative effect to the colony. However, overloading these detoxifying cascades by exposing bees to large quantities of pesticides, such as miticide application to colonies, potentially harms colonies by diminishing their ability to detoxify other natural or synthetic compounds.
Practical application: the key word here is “overload.” Toxin overload can occur from a single pesticide or poisonous pollen, or can be the result of the sum of the additive effects and synergies from any number of plant allelochemicals, pollutants, and miticides.
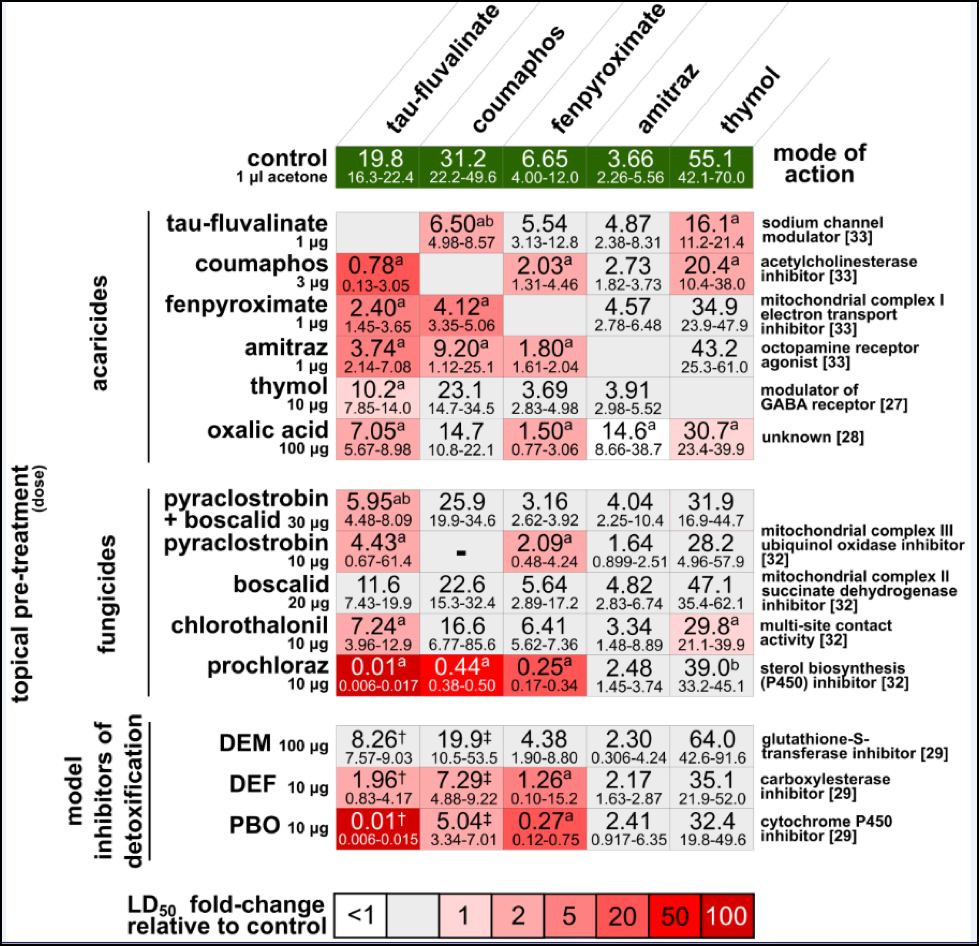
Complicating things is that it’s not just one mode of miticide toxicological action that the bees are forced to deal with—fluvalinate is a pyrethroid, coumaphos is an organophosphate, fenpyroximate is a pyrazole, amitraz is an amidine, and the essential oils have entirely different modes of action (Fig. 2).
Figure 2. Synergism (amplification of toxicity beyond additive effects [20]) of common in-hive miticides with each other and with fungicides. From Johnson, et al (2013) [21].
Conclusion
Back to my leaky boat analogy, the colony has always been challenged by some baseline level of natural toxins, which require some degree of constant bailing. Since the Industrial Revolution, bees have also had to deal with manmade pollution. And in some localities and in some years, those toxins might prove to be too much to deal with, and sink the boat. This occurred long before the invention of synthetic pesticides, and will always be the case.
Once farmers started using insecticides (whether botanical or synthetic), they created “storms” of toxin exposure that would flood the boat, again sometimes overwhelming the hard-working detox/bailing crew [22].
Since the 1940’s the crew has had to deal with the additional toxin/leaks due to the now ubiquitous synthetic insecticides. Each new residue constitutes one more leak in the boat. Not only that, but some classes of pesticides (including miticides and plant allelochemicals) may synergize, meaning that the leaks don’t just add up, but suddenly start gushing dramatically!
And once beekeepers started pouring varroa treatments into their hives, an entirely new baseline of toxin/bailing became necessary. Every bee in the hive was now exposed from birth to death with background residues of miticides, sometimes at levels high enough to sterilize drones and kill developing queens.
Our original question was whether pesticides were responsible for CCD. I couldn’t address that question immediately, since we first had to determine the background level of toxins to which colonies are exposed. Perhaps we can now can start to formulate an answer, from the perspective that today’s honey bee colonies are first burdened with the need to first detoxify the vast array of toxic plant allelochemicals in their diet, as well as any environmental pollutants, plus the additional insult of the beekeeper-applied miticides—all prior to their exposure to any additional agricultural pesticides! Only from that perspective can we realistically measure the impact of “pesticides.” I’ll continue next month…
Addendum: The Beekeepers’ Responsibility
One of the sticking points in the beekeeper’s arguments to the EPA against the misuse of agricultural pesticides is that the majority of commercial beekeepers are themselves guilty of exactly such illegal misapplication of pesticides themselves! This embarrassing fact is frequently brought up by both the EPA and state regulators, and severely weakens our legitimate arguments.
The problem stems from the fact that we have a regulatory system that makes it very expensive to bring legal mite treatments to market. The first major varroacide, Apistan, was much more expensive than MavrikÒ (the off-the-shelf agricultural formulation of the same active ingredient). Although many commercial beekeepers did the right thing and used the expensive strips, most became pesticide scofflaws, and used the illegal treatment to save a few dollars per hive. The same occurred with the next miticide, Checkmite+Ò, but in this case, a number of beekeepers inadvertently poisoned their hives by “off label” use of the cheaper agricultural product.
And then when mites (rapidly) became resistant to that treatment, the beekeeping industry essentially got “thrown to the wolves”—for a time there were simply no effective legal miticides available to commercial beekeepers, forcing them to break the law if they wanted to keep their bees alive! Most of them turned to homebrew treatments based upon various agricultural formulations of amitraz.
The EPA and state regulators generally recognized the fix that we were in, and turned a blind eye to the obvious packets and dabs of chemicals on the top bars, and to the “detects” of DMPF (the degradation residue from illegal amitraz use) in virtually every pesticide residue analysis of commercial combs. Eventually, EPA fired a warning shot across the bow of the beekeeping industry, setting a “zero tolerance level” for amitraz in honey, essentially threatening punitive enforcement action, but had the decency to not follow up.
The situation was resolved this March, when Arysta LifeScience was awarded conditional Section 3 registration of Apivar in the U.S. Such registration now sets a tolerance limit for amitraz in honey, giving commercial (and small-scale) beekeepers two options:
- To do the right thing and use the registered product. Such support of the registrant will allow them to recoup the serious investment that it takes to get a needed product to our industry (the registration process for Apivar has already cost close to a million dollars, and will eventually be more than double that figure! (Even then, it’s a gamble—for example, Hivastan lost), or
- To continue to use cheaper off label homebrews, and then try to wriggle off the hook of potential enforcement action if amitraz residues are found in their honey by claiming that the residues came from the registered product (just in case you’re not sure, this is patently dishonest).
Practical application: If we don’t support the chemical companies when they invest serious money to bring a registered miticide to market (by “cheating” and using the same active ingredient of an agricultural product off label), they will simply abandon us as a market not worth serving in the future!
Keep in mind that Apivar is clearly a better way to use amitraz for mite control than homebrews of agricultural formulations, which contain adjuvants that are toxic to bees. I’ve spoken with Dr. Benoit Siefert of Véto-pharma about the fine points of amitraz and varroa. He explains that the most effective use of amitraz is to partially paralyze the mites, rather than to kill them outright. Such a sublethal effect prevents them from reproducing, which is why the strips must be left in for the full duration (42-56 days). The slow release of the small amount of amitraz keeps residues in the combs and honey to a minimum, and reduces the selective pressure for mites to develop resistance to the active ingredient.
Practical application: Apivar is likely best applied at least two months before you put on honey supers, or the same day that you pull them off.
The flip side is that beekeepers NOW HAVE NO EXCUSE for using “off label” synthetic miticides in their hives. There are now (in addition to the unreliably effective Apistan) four other legal treatments for varroa— HopguardÒ, ApivarÒ, thymol (ApiguardÒ, Apilife VARÒ), and formic acid (MAQS). The latter three all are reliably efficacious at reducing mites to safe levels.
Unfortunately, as Johnson [23] points out:
The regulatory system governing the veterinary use of pesticides in bee hives in the USA may be perversely contributing to the problem. … A change in the regulatory system needs to occur to make effective and safe veterinary pesticides available to beekeepers and to spur research into the effects of candidate compounds on honey bee health.
Call to action: there is yet one more safe, natural, and effective miticide that needs to be registered—oxalic acid. I strongly feel that our industry should put pressure on the EPA to follow its registration in Canada.
The availability of a spectrum of legal treatments nowadays means that the only possible rationalization for not using registered miticides is that the illegal treatments are ‘cheaper” than the legal treatments. If this is an argument for intentionally breaking the law, it simply doesn’t hold water. Not surprisingly, it fails to sway the EPA, which, if it allowed all farmers to use the same argument, might as well throw its hands up in the air (what farmer wouldn’t want to make his own rules and save money?)!
OK, it seems as though I have a predilection for saying the unpopular thing in public, but I’m going to stick my foot in it again! The commercial beekeeping industry needs to start acting like responsible citizens. So long as we keep blatantly using illegal mite treatments, our pleas to regulators to crack down on misuse of agricultural pesticides by farmers are gutted from the get go—if we want to talk the talk, we gotta walk the walk.
Johnson, et al, give us an appropriate scolding:
…beekeepers need to realize that honey bee pests and parasites are community problems, as well as individual problems, and that pesticide labels are crafted to protect the sustainability of pesticides. The use of unregistered products is a serious threat to the beekeeping community and should not occur.
(I am fully aware of the argument that we are not hurting anyone else with what we put into our hives, but that is a deplorably weak excuse when we are producing a food product! Honey’s reputation as a pure and natural product stands to be tarnished by careless beekeepers. I’m also aware that farmers have much more latitude in both choices of pest control products and in application methods. But I don’t want to focus upon excuses—I want us to move forward. Since the off-label use of amitraz is rapidly leading to the development of resistant mites, now would be a good time to rethink the way we do things!)
Crying “Foul”
Commercial beekeepers reply with the argument that there is a lack of “affordable” registered miticides available to our industry. Somehow, I find that that argument falls flat, since I make my own living running a commercial beekeeping operation using only legal miticides (other that the aforementioned oxalic acid). I must compete for almond pollination and honey prices against those beekeepers who unfairly save money by using illegal treatments. That creates an un-level playing field, giving a competitive advantage to the lawbreakers. This is patently unfair, and I cry “foul”!
Doing the Impossible
I often hear that it would be “impossible” to do without the illegal off-label treatments. The reality is that anything seems “impossible” until you just start doing it! Out of frustration, curiosity, and concern about the detrimental effects of residues, I tried managing my operation without synthetic miticides over a decade ago, and never felt the need to go back (I’d use amitraz in a heartbeat if I ever felt the need, but simply haven’t). Since then we’ve tripled the size of our operation to around a thousand hives, take strong colonies to almonds each year, and have sold hundreds of nucs every spring–so much for “impossible”! I’m not suggesting that others give up on synthetics— amitraz is clearly an effective and safe varroacide—but just start using the new legal strips (or at least make the effort to get your local vet to write you a prescription for the off-label use of another amitraz product—this would at least make you “legal”)! Our industry tells the EPA to make farmers do the right thing, even if it costs more, and then pass that cost on to the consumer. Our situation is no different.
CALL FOR ACTION: I call upon the leaders of our industry to set an example and do the right thing…once they do, our industry would no longer have anything to hide, and beekeepers could then start filing the adverse effects reports on pesticides that the EPA is begging for. And if we clean up our own act, we can then demand that farmers do the same!
Acknowledgments
As always, I’m indebted to Peter Borst for his assistance in research. And thanks to Jim and Maryann Frazier, Jerry Bromenshenk, Reed Johnson, Judy Wu, Marion Ellis, Roger Simonds, Diana Cox-Foster, Jennifer Berry, and others whom I’m sure I’ve neglected to mention, for their generosity in discussing these issues with me.
References
[2] Gribble, GW (1996) The diversity of natural organochlorines in living organisms. Pure & Appl Chem 68(9): 1699-1712. (Broken Link!) http://stage.iupac.org/publications/pac/1996/pdf/6809×1699.pdf
[3] Patocka, J, et al (2011) Anatoxin-a(s): natural organophosphorus anticholinesterase agent. Mil. Med. Sci. Lett. 80: 129-139. http://www.vojenskaskola.cz/school/ud/fmhs/research_development/Documents/MMSL_2011_3_5_WWW.pdf
[4] http://www.fda.gov/food/foodsafety/foodcontaminantsadulteration/chemicalcontaminants/ethylcarbamateurethane/ucm078546.htm
[5] Schmeltz, I (1970) Nicotine and other tobacco alkaloids. (Broken Link!) http://wyndmoor.arserrc.gov/Page/1970/3450.pdf
[6] (Broken Link!) http://plantwire.com/plants/Tanacetum/coccineum&img=524935792
[7] Hardstone, MC, and JG Scott (2010) Is Apis mellifera more sensitive to insecticides than other insects? Pest Manag Sci 66: 1171–1180. (Broken Link!) http://entomology-lamp.cit.cornell.edu/scott/159.pdf
[8] Cresswell, JE, et al (2012) Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 115: 365– 371.
[9] Gold, LS, et al (2001) Pesticide residues in food and cancer risk: a critical analysis. In: Handbook of Pesticide Toxicology, Second Edition (R. Krieger, ed.), San Diego, CA: Academic Press, pp. 799-843 (2001). http://potency.berkeley.edu/text/handbook.pesticide.toxicology.pdf I highly recommend this read!
[10] Després (2007) Op. cit.
[11] Mao W, et al. (2009) Quercetin-metabolizing CYP6AS enzymes of the pollinator Apis mellifera (Hymenoptera: Apidae). Comp Biochem Physiol B Biochem Mol Biol 154: 427–434.
[12] Mao, W, et al (2011) CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera) http://www.pnas.org/content/early/2011/07/20/1109535108.full.pdf+html
[13] Frazier, J, et al (2012) Pesticides and their involvement in colony collapse disorder. http://www.extension.org/pages/60318/pesticides-and-their-involvement-in-colony-collapse-disorder A “must read”!
[14] Johnson, RM, et al (2010) Pesticides and honey bee toxicity – USA. Apidologie http://entomology.unl.edu/faculty/ellispubs/Pesticides.pdf
[15] https://scientificbeekeeping.com/the-arsenal-our-choice-of-chemical-weapons/
[16] Pettis, J & D vanEngelsdorp (2010) Reduced longevity of adult worker bees related to coumaphos exposure in wax during larval and pupal development. http://www.ars.usda.gov/is/br/ccd/ccdprogressreport2010.pdf
[17] Wu, Judy Y, et al (2012) Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (Microsporidia) infection. Journal of Invertebrate Pathology 109: 326–329.
[18] Johnson, Reed M, Marion D. Ellis, Christopher A. Mullin, Maryann Frazier (2010) Pesticides and honey bee toxicity – USA. Apidologie 41: 312–331. Available online at: www.apidologie.org
[19] Boncristiani, H, et al (2012) Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. Journal of Insect Physiology 58: 613–620.
[21] Johnson (2010) Op. cit.
[22] Butler, CG, et al (1943) Experiments on the poisoning of honeybees by insecticidal and fungicidal sprays used in orchards. Annals of Applied Biology 30(2): 143–150. “Losses of bees by poisoning have been greatly increased in recent years by the growing practice of applying insecticidal and fungicidal sprays to fruit trees… When arsenical sprays alone are applied to trees, particularly if to the open blossom as is sometimes the case with gooseberries and cherries, severe poisoning from the pollen may be expected.”
[23] Johnson (2010) Op. cit