Sick Bees – Part 14: An Update on the “Nosema Cousins”
CONTENTS
Worldwide Status and Distribution
What if You’re Dealing with N. apis?
Randy Oliver
ScientificBeekeeping.com
In my last article, I described how to quickly sample for nosema. So what do the spore counts actually mean as far as colony health is concerned? I wrote an article a little over two years ago with the tongue in cheek title “Nosema ceranae: Kiss of Death, or Much Ado about Nothing.” Well, N. ceranae is still an enigma, but it appears that the answer lies somewhere in between.
Dr. Mariano Higes (2005, 2006) was the first to raise the flag to alert beekeepers worldwide that a new species of nosema had invaded Europe, and appeared to be the cause of the unusual colony collapses that plagued Spain (a major beekeeping country) in 2003 and 2004. Then in 2007, just as Colony Collapse Disorder was rampaging through our own bee operations, we found out that Nosema ceranae had somehow spread throughout the U.S. right under our eyes!
Drs. Diana Cox-Foster and Ian Lipkin (2007) then published a paper suggesting that a newly-described virus was involved in CCD, but later research indicated that IAPV wasn’t the only culprit, leaving N. ceranae as a leading suspect.
Shortly afterward, Higes (2008) described in great detail the progression of N. ceranae infection (in his Spanish apiaries) through four stages: Asymptomatic, Replacement, False Recovery, and finally the dreaded Depopulation. The logic, the numbers, and the devastating final result were all clear and compelling. The specter of N. ceranae ravaging our hives resulted in unnerved beekeepers boosting the sales of fumagillin to the point that supplies ran short.
I had never previously worried about nosema, but I pulled out a microscope and found out that N. ceranae was indeed widespread in my operation. I ran trials, and found out that the danged parasite could flourish despite being drowned in fumagillin (Oliver 2008a), but more surprisingly, that colonies here at Comedy of Errors Apiaries thrived despite exhibiting spore counts in the millions. To try to reconcile the differences between the very different outcomes of N. ceranae infection in my operation with those reported for Spain, I began an ongoing correspondence with Dr. Higes, which continues to this day.
To be frank, some other Spanish researchers dispute Higes’ conclusions (debate leads to better science), so I have often questioned and challenged him on details of methodology and interpretation, which he and his team of collaborators have generally clarified with additional research. In this series of articles I will be citing a number of the Higes team’s papers, since they have clearly led the pack in N. ceranae research, meticulously investigating nearly every aspect of this pathogen’s effects upon bees.
I’ve previously written at length about N. ceranae in my “Nosema Twins” series (all available at ScientificBeekeeping.com), but feel that there has been so much recent research completed that it would benefit the reader for me to write a digest of our current state of knowledge. I’ve scoured the literature for every relevant research paper (including a number still in press), and have discussed as well current findings with many of the world’s nosema researchers. I wish that at this time I could say that I have the answers to all your questions about Nosema ceranae, but unfortunately, in many aspects this parasite still remains an enigma.
WORLDWIDE STATUS AND DISTRIBUTION
Nosema ceranae has now spread into the European honey bee populations of most areas of the world, roughly concurrent with the spread of varroa (and its altering of virus dynamics), which greatly confuses analysis of the effect of these two novel parasites upon bee health. It is difficult to tell in which countries N. ceranae has already reached equilibrium, and in which it is still invading.
Since the first invasive wave of a novel parasite into naïve hosts is generally that most damaging, it would be helpful to know when ceranae actually arrived in various countries. For example, we know from analysis of archived bee samples that N. ceranae has been present on the East Coast for at least two decades (Chen 2008). Unfortunately, any initial effects of its invasion may have been masked by our focus upon the massive impact of the arrival of varroa at about the same time.
Since no one was looking for N. ceranae in the U.S. until 2007, we obviously didn’t start studying it until long after it was well established and likely homogenized throughout the bee population via migratory beekeeping practices. And it is also likely that by the time we started studying the impact of N. ceranae upon the health of colonies, natural selection may have already weeded out the bees least tolerant of the emergent pathogen.
In Europe, however, N. ceranae only recently invaded bee populations already suffering from varroa and viruses, miticide failure and comb contamination, extreme weather events, plus changes in agricultural practices and pesticide use—the combination of which likely factor into colony losses in that region.
In a fresh study (Botías 2011), the Higes team analyzed archived Spanish honey samples (frozen) and adult bee samples (in alcohol) dating back to 1998. They found that N. ceranae first appeared beginning in 2000 and increased in prevalence through 2009 (the latest samples analyzed), concurrent with a decrease in the prevalence of N. apis. It is noteworthy that Spain concurrently suffered from devastating drought during much of that period, which led to serious colony stress.
N. ceranae is still in the process of extending its range worldwide, and appears to be most successful in warmer climates. It is of interest that in varroa-free Australia, its invasion does not appear to be causing significant colony losses. Interestingly, although it is well-established in Canada, it is not yet common in some northern European countries, but this may be due to restrictions upon bee imports (Fries 2010).
N. ceranae is widely distributed throughout the U.S., but surprisingly, there were great differences in the percent of colonies infected in a recent state-by-state survey (Fig. 1).
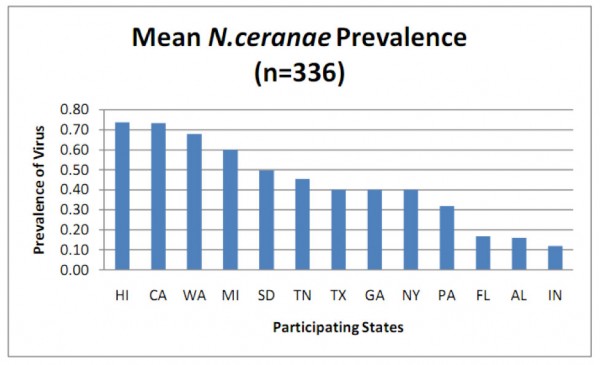
Figure 1. Prevalence (percent of samples infected) of N. ceranae in various states as determined by PCR analysis (more sensitive than spore counts) of aggregate samples collected from 8 randomly selected colonies per apiary, 4 apiaries per state. Note that in some states over 70% of samples were infected! From Rennich, K, et al (2011) 2010-2011 National Honey Bee Pests and Diseases Survey Report.
CERANAE VS. APIS
In a widely cited paper by Martín-Hernández (2007), her arresting graph of nosema positive samples over time clearly shows a definite shift over the period from 1999 to 2005—there initially were only spikes in spring and fall (ostensibly from apis), transitioning to nearly 100% of samples being positive every month of the year (due to ceranae). Of note is that her data has an inherent bias, in that the samples were voluntarily sent to the lab by beekeepers for diagnosis of problems, suggesting that the data may reflect the change in nosema loads in sick hives. Also of note, is that despite this graph being widely cited, it is often misunderstood—it did not plot spore levels, but rather only the yes/no detection of nosema spores.
What the graph did strongly indicate was that N. ceranae rapidly and thoroughly invaded Spain over a period of only a few years! This initial finding has now been confirmed by Botías (2011). Likely, a similar phenomenon occurred in the U.S., since Chen (2008) found N. ceranae to already be widespread in archived U.S. bee samples dating back to 1995.
The general trend appears to be that N. ceranae now predominates in warmer countries, whereas N. apis is better adapted to colder areas. It has been often stated that N. ceranae has displaced N. apis, but more careful analysis suggests that that may not actually be the case!
When Dr. Robb Cramer asked me in 2007 to send him infected bees so that he could culture pure N. ceranae, he found that the samples often contained some N. apis as a “contaminant.” In Dr. Diana Cox-Foster’s (2007) analysis of CCD colonies, they also found both species of nosema. Later studies by Bourgeois (2010) and Runckel (2011) of commercial operations in the U.S. also found N. apis, but in far fewer hives than its cousin, only in spring and/or fall, and notably, at much lower spore levels than N. ceranae.
The differences between the detectability of the two nosema species (N. apis typically produces much lower spore counts and is generally only seen in spring and fall) may lead “to an increased chance of detecting N. ceranae over N. apis, which could have biased the impression that N. apis has been displaced” (Higes 2010).
So, has ceranae actually displaced apis, or have we merely been overlooking its cousin? In order to answer that question, Dr. Raquel Martín-Hernández (2011) carefully analyzed over 2000 bee samples from all across Spain. She found ceranae and apis coexisting throughout country, with ceranae clearly predominant (in roughly 40% of hives), apis hanging in there (in up to 15%), and occasional mixed infections (below 7%). She also found that infection by ceranae was favored in hotter areas of the country, whereas apis succeeded better where winters are colder.
I’m seeing similar indications from other countries (e.g., Gisder 2010), which are appearing to confirm that apis is the more cold-adapted species. As far as seasonality, Martín-Hernández found apis only in the spring and fall, whereas ceranae could be found all year, and notably, once ceranae infects a colony, it almost always persists (detectable with PCR, even if not obvious via spore counts).
Practical note: these studies indicate that N. ceranae remains present as an infection in a colony throughout the year, even if it is not detectable by microscopy. But we don’t know whether these inapparent infections affect colony health.
I found one last study to be of special interest: Dr. Judy Chen (2009) looked at nosema invasion from the other direction—in a turn of the tables, N. apis appears to have been introduced from the Western honey bee (Apis mellifera) into the Eastern honey bee (Apis cerana) in Asia, and is now an emergent parasite in that species, which had historically been infected only by N. ceranae! She analyzed bee samples from China, Taiwan, and Japan. Her findings:
“N. apis was detected in 31% of examined bees and N. ceranae was detected in 71% of examined bees and that the copy number of N. ceranae was 100-fold higher than that of N. apis in co-infected bees, showing that N. ceranae is the more abundant of two Nosema species in the Eastern honey bees.”
This study suggests that N. apis can not only hold its own against N. ceranae, but can actually invade into ceranae’s turf! Interestingly, in the Eastern honey bee, despite its long coevolution with N. ceranae, ceranae still produces higher spore counts than its invading cousin.
COINFECTION
This brings up the question of what happens when bees are infected simultaneously by both species of nosema? Dr. Zachary Huang (pers comm) found that in both cage trials and field observations that longevity was substantially shorter for coinfected bees as opposed to those infected by either species of nosema alone (unpublished data).
Note that in Cox-Foster’s (2007) CCD study that they found “a trend for increased CCD risk in samples positive for N. apis” (100% of CCD colonies tested positive for ceranae and 90% for apis, but remember that apis is easy to miss when samples consist of house bees). As Jim Fischer noted in a post to Bee-L, “What was striking was that every hive showing CCD symptoms tested positive for BOTH Nosema apis and Nosema ceranae, and this correlation was better than the correlation between CCD and IAPV that was the focus of the paper.”
These findings leave me very curious about the impact of coinfection by two nosema species upon colony health!
SEASONALITY
Spore counts of N. ceranae generally reach a peak in May, then drop spontaneously during summer, and may spike sporadically in fall and winter. But there is more to the picture than this. Dr. Ingemar Fries (2010), who has studied nosema for decades, explains thusly:
“The typical pattern for N. apis infections in temperate climates is low prevalence or hardly detectable levels during the summer with a small peak in the fall. During the winter there is a slight increased prevalence with a large peak in the spring before the winter bees are replaced by young bees… The pattern is similar both in the southern and northern hemisphere… Unfortunately, very few data exist for N. apis on the seasonal prevalence from tropical or subtropical conditions. The only published year round sampling under conditions where bees could fly all year round, revealed detectable levels of N. apis with no seasonal pattern of prevalence.”
Along that line, Dr. Denis Anderson in Australia (pers comm) tells me that, “there are also many unseasonal occurrences of N. apis — I get many samples sent in in the mid summer here that are loaded with N. apis.” This could well be happening in the U.S., where, as far as I can tell, there have been few studies on N. apis in warmer areas, other than the fact that it was commonly found in package bees produced in the southern states.
Practical application: we need to learn more about the prevalence and seasonality of N. apis in the warmer parts of our country!
I’ve now seen data and presentations on N. ceranae seasonal prevalence from researchers from all over the world. Since a picture is worth a thousand words, I’ve summarized them in a crude graph below (Fig. 2).
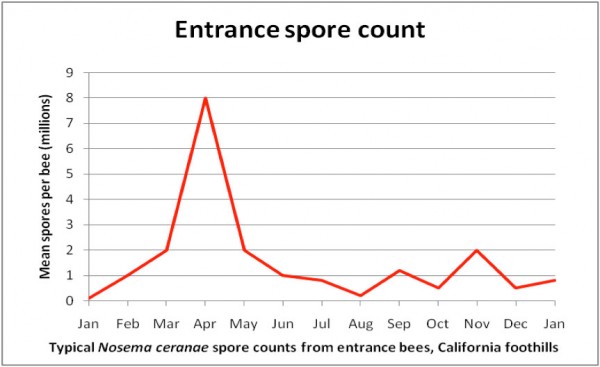
Figure 2. A generic graph of typical N. ceranae spore counts over the course of the year in my operation. Important note: Counts of house bees would follow the same trend, but at much lower levels. The late-season spikes are often sporadic flare ups that spontaneously “go away.”
Practical application: It is not unusual to see high nosema spore counts in April and May. Counts will typically drop in summer whether you treat or not. I’ll cover treatments in a subsequent article.
But new technology is showing something surprising about nosema sampling—that spore counts do not necessarily reflect degree of actual nosema infection (Meana 2010)! Look at the following graph (Fig. 3), from a recent nationwide study of pathogens in U.S. bees—instead of measuring spore counts, the blue bars indicate the percentage of colonies infected by N. ceranae as determined by DNA analysis (PCR).
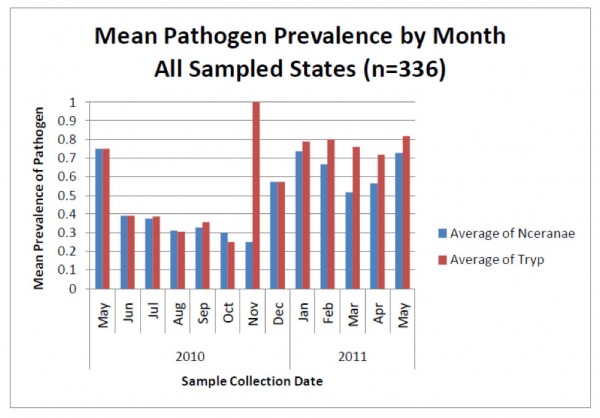
Figure 3. The blue bars indicate the percentage prevalence of N. ceranae in sampled colonies (e.g., 0.7 = present in 70% of hives). Note that even though spore counts suggest that N. ceranae disappears for much of the year (previous graph), a substantial proportion of colonies actually remain infected to some degree by the parasite. Also note how closely the coinfection with another intestinal parasite (the presumably opportunistic trypanosomes) tracks nosema infection. No one is sure whether there is a causal relationship, or whether the simple explanation is that both parasites flourish in stressed bees. Graph from Rennich, K, et al (2011) 2010-2011 National Honey Bee Pests and Diseases Survey Report.
As opposed to the above graph, Runckel (2011) also measured the amount of nosema DNA in samples, which presumably correlates with the intensity of the infection. They found high levels of N. ceranae transcripts in midsummer, at a time when spore counts are generally quite low (Fig. 5)! Their data indicated that N. apis was only present in spring and fall (which does correspond to spore counts). Go figure!
So what’s up with high levels of N. ceranae DNA transcripts without correspondingly high spore counts? No one to my knowledge has answered that important question. What we do know is that N. ceranae can exist in the vegetative stage for a while before it produces spores (Martín-Hernández 2009). But we’re not clear on to what extent N. ceranae produces “autoinfective spores,” as opposed to the “environmental” spores that are discharged into the gut contents (Cali 1999), and whether such autoinfective spores show up under microscopy. What is clear, however, is that N. ceranae appears to be able to reproduce within a bee without producing spores that are observable by microscopy.
Practical note: although N. ceranae spore counts may disappear in summer, DNA analysis indicates that the bees may still be infected. This is something of a mystery, as the bee population turns over rapidly during the summer, suggesting that N. ceranae is somehow infecting new bees without spores being evident!
So the next question is, is an infection by N. ceranae more pathogenic than one by N. apis? Although some initial cage trials indicated extreme virulence for the new nosema, trials in which bees were allowed to feed upon natural pollen generally found that both species affect bee longevity about the same (Forsgren 2010, Porrini 2011, Huang pers comm) despite the fact that spore levels get much higher with N. ceranae.
Take home: We clearly still have lots to learn about N. ceranae! It does not appear to cause rapid death of well-fed bees. The inapparent summer infections are puzzling.
So what’s the cause of the seasonality of nosema spore counts? With N. apis it is presumed to be due to the requisites of transmission via dysentery by infected bees in the hive during the winter and colony nutritional stress, and limited by its sensitivity to high temperature. Martín-Hernández (2009, 2010) demonstrated that N. apis can only grow in a narrow range of temperature (about 33°C). N. ceranae, on the other hand, grows readily over a range from 25°C to 37°C. However, N. ceranae spores are surprisingly susceptible to chilling (Fries 2010), which may limit their infectivity at lower temperatures.
Studies from a number of countries coinfected with both of the nosema cousins suggest that N. apis will continue to be the historical problem during winter and spring, with typical fall and spring spikes, whereas ceranae will be more prevalent in warmer climes, present throughout much of the year, spiking in late spring (perhaps tracking pollen flows), and then again sporadically in fall through winter.
Take home: if Nosema apis was a problem in your area prior to the invasion of N. ceranae, it may still contribute to colony health issues during the fall and spring!
SAMPLE INTERPRETATION
It would sure be easier if there were a simple sampling protocol that everyone could follow, and if there were clear treatment (or worry) thresholds based upon nosema spore counts, as there are for varroa (Fig. 4), but alas, I’m sorry to say that there aren’t.
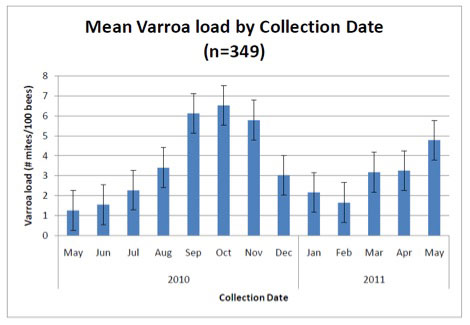
Figure 4. Average varroa infestation rates from 2700 colonies in 13 states (many of which received mite treatments). Sampling for varroa infestation level is relatively straightforward and simple to interpret. Typical treatment thresholds are below 5 mites per 100 bees. Graph from Rennich, K, et al (2011) 2010-2011 National Honey Bee Pests and Diseases Survey Report.
Unlike sampling for varroa, which are easily seen with the naked eye, sampling for nosema requires either a microscope or laboratory apparatus that can perform PCR. However, a number of researchers (Meana 2010, Bourgeouis 2010, Traver 2010) have demonstrated that spore counts alone do not give an accurate picture of the actual degree of infection. Unfortunately, as far as assessment methods available to Joe Beekeeper, spore counts will have to suffice as a surrogate measure of the actual degree of infection (Fig 5).
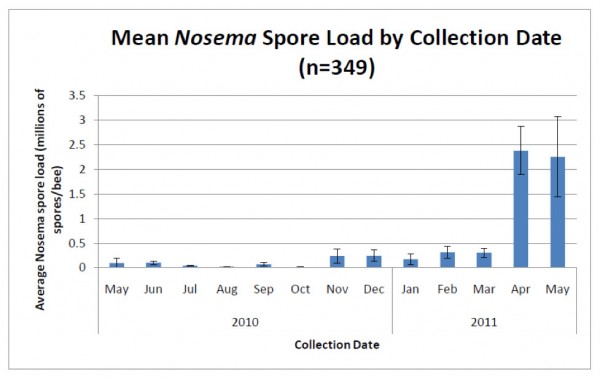
Figure 5. Average nosema spore counts from the same 2700 hives. Note the typical huge spike in spore counts (predominantly from N. ceranae) in spring, and then again lesser spikes in fall and winter. Important note: these spore counts were from samples of bees from brood frames—counts from entrance bees would likely be several times higher (compare to Figure 2). Graph from Rennich, K, et al (2011) 2010-2011 National Honey Bee Pests and Diseases Survey Report.
That said, let’s return to sampling for a bit. If you want to find spores, then sample older bees, such as foragers at the entrance (Meana 2010)—spore counts will typically be about 10 times higher in older bees, since it takes a while for the infection to build up in a bee (Smart 2011). He found that in infected colonies with a background spore count of 0.5-1M in bees from under the inner cover, almost no bees younger than 12 days old contained spores (at least detectable by microscopy).
This is not at all surprising, since El-Shemy (1989) found the same to be true for N. apis—spore counts were an order of magnitude higher in bees from the entrance. Indeed, he suggested that it was best to sample exiting bees at the entrance, since returning bees have likely defecated. The magnitude of the spore counts from an infected colony generally increases in samples (in order from lowest to highest), of bees from the broodnest, outer areas of the cluster, entrance bees, exiting foragers, returning foragers.
Both El-Shemy and Higes (2008) found that the best indicator of degree of infection was to squash bees from an entrance sample one at a time in order to determine the percentage of bees infected. My own sampling of sick colonies supports this recommendation. But in reality, few of us have time to squash dozens of bees one at a time for each sample—so I won’t even suggest that you go there!
The next best method may be to do a spore count for a pooled sample of 50 bees from the entrance (but don’t forget that even one or two highly-infected bees can greatly skew the count). In practice, however, it is often danged difficult and time consuming to collect 50 entrance bees, even if you use a special vacuum (Oliver 2008b), especially in cool weather or from sick colonies with few foragers.
For this reason, many researchers simply take standardized samples of bees from under the cover, or from an outside comb. There is support for this, as Gajda (2009) found that although spore counts were much higher in entrance bees, the relative proportion of infected bees was similar in samples taken from an outside comb.
Practical application: If you want to find out whether N. ceranae is present to any significant extent in your operation, sample bees from the entrance. If you want to know if the infection is serious, sample house bees from under the cover.
If you are curious as to whether you have gotten old or young bees in your sample, here is an easy general observation that I’ve made: since only nurse bees normally eat pollen, they are the only ones that will have it in their guts (duh). But my point is, that this is really easy to use that pollen as an indicator of bee age if you use the ziplock bag method for processing samples (see my previous article, and Fig. 6).

Figure 6. How to tell if your sample contains young or old bees. (Left photo) when you crush samples of nurse bees in a ziplock bag, and then mush them in water, the fluid will typically turn opaque yellow (since the guts of nurse bees are full of pollen). (Right photo) on the other hand, the fluid from the guts of entrance bees will typically be a tan/gray color (since foragers and guards don’t eat pollen).
WHAT IF YOU’RE DEALING WITH N. APIS?
Oh, that it were only so simple as dealing with only one nosema, but the previously cited studies suggest that many of us actually may still have N. apis popping up in fall and early spring. To make things even harder, spore counts of N. apis, on a per bee, or per pooled sample basis, are generally only a fraction (about 1/10th, as best I can tell from previous studies) of what we see with N. ceranae. But it also appears that an infection by N. apis at that low level can be as serious as an infection by N. ceranae at a much higher spore count!
Important note: Martín-Hernández (2011) easily found N. ceranae in samples of either foragers or house bees, whereas she only found N. apis in foragers and drones. So if N. apis is your concern, then you should take entrance samples! N. apis infection may be serious at a much lower spore level!
SEASONALITY
The other consideration is that you must put any spore count into the context of time of year, the climate that your bees are in, the nutritional status of the colonies, and especially the load of other pathogens. I will discuss these points in the next article.
In cold climates, nosema management may have other considerations. Hedtke (2011) performed a detailed 6-year study of 220 hives in Germany, and (surprisingly) found that “No statistical relation between N. ceranae detection in autumn and the following spring could be demonstrated, meaning that colonies found to be infected in autumn did not necessarily still carry a detectable infection in spring, and colonies which developed a detectable infection over winter had not been detectably infected in autumn.” So much for careful sampling!
RECOMMENDATIONS
Heck, I’d be crazy to stick my neck out and give any recommendations! So let’s look at what sort of nosema levels are involved in crashing colonies. The CCD colonies analyzed by Cox-Foster (2007) had mean spore counts in the range of tens to hundreds of millions from broodnest samples! Is it really any surprise that those colonies collapsed? The house bees in Higes’ (2008) winter-collapsing colonies hit 20M before they went down (field bees hit 50M), but those that collapsed in summer only hit 3M.
But note that in the U.S. survey graph above, that 2M was the average spore count across the U.S. in April and May of this year, yet I’m not hearing of massive colony collapses, despite very poor conditions in many states.
In my own California foothill operation (we get snow during the winter, and move to almonds in February), it is not unusual to see entrance spore counts in May in the millions or tens of millions, but they generally drop during summer, provided that colonies are not stressed by other factors. Entrance counts during summer and fall are typically in the zero to 5M range (25 spores per field of view if you follow the protocol in my previous article—I’ll call these FOV counts (Oliver 2008c)). I have not looked at near as many samples of house bees, but counts are generally zero to a fraction of a million, even in colonies running at 10M at the entrance.
I am by no means suggesting that you follow my lead, but I simply no longer worry about high spore counts in spring, as they generally spontaneously drop later in the season, and I haven’t experienced winter losses associated with N. ceranae (unless I’ve intentionally inoculated the hives with viruses). However, I do keep my mite levels down, and feed pollen supplement to maintain good nutrition if necessary. And I monitor nosema levels throughout the year so that I don’t get blindsided!
I’ve never treated for nosema (except in experiments), yet have not experienced colony collapses since 2006. But I’m not saying that you have no reason for concern—I will be writing about a trial in which I did compare survival of treated vs. untreated colonies that had virus issues, and fumagillin appeared to help.
I’d be concerned if counts for house bees got above 5 per FOV at any time, although I know several large commercial beekeepers who routinely ignore such counts with no dire consequences so far. I just checked a number of samples of house bees today (late October), and they ran from zero to 2 spores per FOV, despite there often being counts of 100-200 per FOV of entrance samples this spring.
In some operations where N. ceranae apparently got out of hand, treatment and comb sterilization seemed to help. However, in other operations with sky-high spore counts in spring, lack of treatment did not result in any noticeable problems. Due to these huge discrepancies, it is confoundingly difficult to come up with recommendations. However, the more beekeepers who start tracking spore counts, the more we will learn about appropriate treatment decisions.
If you are in an area with a long, cold winter which keeps the bees confined, you may be dealing with Nosema apis, for which the economic threshold of 1M (5 per FOV) for house bees has been well established.
Practical application: since spore counts for N. apis generally only reach levels about 1/10th of those for N. ceranae, you’d be wise to ask your local university determine which nosema species you’re dealing with, since it follows that the economic threshold for treatment for N. apis may be far less than that for N. ceranae.
I will continue this review of N. ceranae in the next issue, including treatments, and its relationship to colony mortality and honey production.
ACKNOWLEDGEMENTS
Thanks to you, my readers! It just occurred to me that I’ve recently passed the 5 year mark in writing for ABJ, and it’s been one wild ride! If I had any idea what I was getting into, I would probably have chickened out. But your feedback and appreciation keep me going—my motivation is simply the gratification that I get from sharing what I’ve learned with other beekeepers. Your donations also allow me to perform the sort of quick and dirty research necessary to answer burning questions. I am constantly on the learning curve, and greatly appreciate hearing information that is relevant to better bee management—feel free to contact me (no beginners questions please) randy@randyoliver.com.
As always, Peter Loring Borst has helped me greatly with research. I thank Dr. Mariano Higes for his patience in discussing his research. Dr. Steve Pernal and Ingemar Fries have been gracious with their time. I also thank all the other nosema researchers who have patiently answered my questions.
REFERENCES
Botías, C, et al (2011) The growing prevalence of Nosema ceranae in honey bees in Spain, an emerging problem for the last decade. Research in Veterinary Science (in press).
Bourgeois, AL (2010) Genetic detection and quantification of Nosema apis and N. ceranae in the honey bee. Journal of Invertebrate Pathology 103: 53–58.
Cali, A and PM Takvorian (1999) Developmental morphology and life cycles of the microsporidia. P. 121. in Wittner, M and LM Weiss, eds. The Microsporidia and Microsporidiosis.,American Society for Microbiology.
Chen, Y.P., et al (2008). Nosema ceranae is a long-present and widespread microsporidian infection of the European honeybee (Apis mellifera) in the United States. J Invertebr Pathol 582 97: 186–188.
Chen, YP, et al (2009) Asymmetrical coexistence of Nosema ceranae and Nosema apis in honey bees. Journal of Invertebrate Pathology 101 (2009) 204–209.
Cox-Foster, DL, et al. (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318(5848): 283-287.
El-Shemy, A.A.M. and RS Pickard (1989) Nosema apis Zander infection levels in honeybees of known age. J. Apic. Res. 28 (2), 101–106.
Forsgren, E, and I Fries (2010) Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Veterinary Parasitology 170: 212–217.
Fries, I (2010) Nosema ceranae in European honey bees (Apis mellifera). Journal of Invertebrate Pathology 103: S73–S79. (Broken Link!) https://bienenkunde.uni-hohenheim.de/uploads/media/Nosema_ceranae_in_European_honey_bees__Fries.PDF
Gajda, A (2009) The size of bee sample for investigation of Nosema sp. infection level in honey bee colony. http://www.coloss.org/publications/Nosema-Workshop-Proceedings.pdf
Gisder S, et al. (2010) Five-year cohort study of Nosema spp. in Germany: does climate shape virulence and assertiveness of Nosema ceranae? Appl Environ Microbiol 76: 3032–3038.
Hedtke, K, et al (2011) Evidence for emerging parasites and pathogens influencing outbreaks of stress-related diseases like chalkbrood. Journal of Invertebrate Pathology 108:167–173.
Higes, M (2010) Nosema ceranae in Europe: an emergent type C nosemosis. Apidologie 14(3): 375 – 392.
Higes, M., et al (2005) El síndrome de despoblamiento de las colmenas en España. Consideraciones sobre su origen. Vida Apícola 133: 15–21.
Higes M, et al (2006) Nosema ceranae, a new microsporidian parasite in honeybees in Europe, Invertebr Pathol. 92(2):93-5.
Higes, M, et al (2007) Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J Invertebr Pathol. 94(3):211-7
Higes, M, et al (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol 10: 2659–2669.
Martín-Hernández, R, et al (2007) The outcome of the colonization of Apis mellifera by Nosema ceranae. Applied and Environmental Microbiology http://aem.asm.org/cgi/content/abstract/AEM.00270-07v1
Martín-Hernández, R., et al (2009) Effect of temperature on the biotic potential of honeybee microsporidia. Applied and Environmental Microbiology 75(8): 2554–2557.
Martín-Hernández, R, et al (2011) Microsporidia infecting Apis mellifera: coexistence or competition. Is Nosema ceranae replacing Nosema apis? Environmental Microbiology and Environmental Microbiology Reports (in press).
Meana, A, et al (2010) The reliability of spore counts to diagnose Nosema ceranae infections in honey bees. Journal of Apicultural Research and Bee World 49(2): 212-214.
Oliver, R (2008a) A test of the drench method for nosema treatment. ABJ 148(10): 917-923.
Oliver, R (2008b) The Suck-a-Bee. ABJ 148(8): 719-721.
Oliver, R (2008c) Field of View Count Correlation. https://scientificbeekeeping.com/field-of-view-count-correlation/
Pajuelo, AG, et al (2008) Colony losses: a double blind trial on the influence of supplementary protein nutrition and preventative treatment with fumagillin against Nosema ceranae. Journal of Apicultural Research 47(1): 84-86.
Porrini, MP (2011) Nosema ceranae development in Apis mellifera: influence of diet and infective inoculum. Journal of Apicultural Research 50(1): 35-41.
Rennich, K, et al (2011) 2010-2011 National Honey Bee Pests and Diseases Survey Report
http://www.aphis.usda.gov/plant_health/plant_pest_info/honey_bees/downloads/2010-2011-Limited_Survey_Report.pdf
Runckel C, et al. (2011) temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS ONE 6(6): e20656.
Smart, M.D. and WS Sheppard (2011), Nosema ceranae in age cohorts of the western honey bee (Apis mellifera). J. Invertebr. Pathol. doi:10.1016/j.jip.2011.09.009
Traver, B.E., and Fell, R.D. (2011) Prevalence and infection intensity of Nosema in honey bee (Apis mellifera L.) colonies in Virginia. J Invertebr Pathol 107 (1):43-49.



