Update 28 Oct 2018
Beekeeper Nick Kingan let me know that there’s a nice livestock syringe available from Tractor Supply that can be adjusted to dispense 5 mL per squeeze. I find the squeezing of this sort of syringe to be tiring to my hand if I’m treating a large number of hives, but it may be handy for those with just a few hives, since it helps to dispense the correct dose.
Update 20 Sept 2017
There are YouTubes being circulated recommending using a heat fogger to apply oxalic acid. Retired chemist Dick Cryberg, who is a very sharp guy whose observations I deeply respect, posted the following to Bee-L:
I tried fogging with 10% oxalic acid dissolved in water. I fogged ten five over five deep nucs once a week with 0.6 g of oxalic acid
dihydrate for each five deep for eight consecutive weeks this summer. I had ten nucs untreated side by side for controls. The single highest
after treatment mite count was from a treated nuc determined by alcohol wash at 3%. I saw exactly zero evidence that I killed any significant number of mites. Overall the treated hives and controls had equivalent mite counts. All were at 0 to a bit over 1% mites at the start. I dosed as high as 2g/ five deep nuc and showed no adverse effects other than a few burned bees that hit the fogger screen. Fogging ran from mid June
to late July. All were treated earlier in the spring to drive mite counts to about zero with apivar. The slow build up by early August is because I run Minnesota Hygienic queens which do a fair job of containing mite populations.I was pretty careful in the experiment. I spent several hours fooling with the fogger to figure out how to deliver a consistent dose. I had to drill a hole in the top of the handle and push down the trigger fully down with a screw driver after each shot to get consistent volume each time. I also had to pull the trigger as fast as possible to get consistent volumes. I showed, by capturing and analyzing the fog that oxalic was surviving the fogging experience at least partly intact. My capture was under 100% and analysis showed a recovery of 60% so I feel was I was not
decomposing enough to matter.I also waited 20 seconds between trigger pulls to allow full heat recovery in the coil. Even then a lot of what came out the spout was liquid that ended up on the bottom of the nuc. I found the process tedious and not really all that fast at over three minutes per nuc each treatment.I have seen the you tube vids and seen much discussion on fogging. I am the first, as far as I can tell, to ever do before and after mite counts and include controls of any type. At this point I view all such claims as pure snake oil of the usual value that snake oil typically has but am open to being proven wrong. I am a bit leary of firing alcohol due to flammability althou no one has reported a fire issue. [I would expect a fog of alcohol in air to be highly explosive, but I don’t have a heat fogger with which to test. If any reader has tested this, please let me know]
Also, oxalic acid will react very rapidly with alcohol make the ester and the ester will very rapidly decarboxylate at temps as low as 100 deg C to ethyl formate which is not going to kill mites. If you are going to fog ethanol solutions you probably need to make fresh solution just prior to fogging to avoid the inevitable ester formation that is going to happen on storage even at room temp. This chemistry is not a problem in water solution but needs to be considered in alcohol or particularly glycerin which some are using.
Update 18 March 2017
When OA dribbling package bees (or perhaps any bees), I’ve received a report from a good source that the bees tolerate the dribble better if they are full of nectar or sugar syrup–presumably because they are then less prone to imbibe the OA syrup.
Update 24 Jan 2017
There has been lots of response to my article on OA/gly in ABJ (soon to be posted to this website). I’m in communication with EPA to get this application method approved.
Update 22 Dec 2016
Be sure to check my article in Jan ABJ on OA/glycerin https://scientificbeekeeping.com/oxalic-shop-towel-updates/. I’ll try to post soon.
In response to questions about adverse effects on queens:
The evidence that I’ve seen to date indicate that vaporization does not have serious effects on the queen. On the other hand, there are indications that repeated dribble may.
In our operation, we don’t notice any effect on queens from spring treatment of nucs, or from one-time fall treatment of colonies with dribble (but we haven’t run controlled trials to compare rates of winter queen failure).
My GUESS is that repeated exposure to HIGH concentrations of OA likely is stressful to queens (for example, when applying vapor at 5-day intervals to obtain good efficacy in colonies with brood). Worker turnover in colonies is rapid in summer, so adverse effects on workers from cumulative exposure may not be as noticeable as with queens. Colonies thrive during warm weather under chronic exposure via OA/glycerin, but a beekeeper in Chile says that use during damp winter weather can result in wing burn off in queens.
Take home–we don’t yet know enough to give a definitive answer.
Update 16 June 2016
I recently received an email from a Northeastern beekeeper, Erik Donley, about his experience with applying oxalic vapor to newly-hived package bees. Excerpts follow:
* I installed 10 x 3LB packages (from OHB) in single deep hives. (April 17th) All but one hive had fully drawn out comb. (The 1 was starting with 1 drawn comb and 9 bare foundations)
* On the 8th day after installation I administered the OA treatment. I vaporized approx .75g (between 1/4 and 1/8 teaspoon) of OA into each single deep hive. (I felt that was a reasonable dose given a full 2 deep hive takes roughy 2g)
* I checked the hives 4 days after and everything seemed fine. All continued to have laying queens with solid brood patterns, there were no issues with absconding, or mutiny vs the queen.
* Since installation, the hives have moved to multiple locations across Northern Minnesota and Wisconsin and have been exposed to a variety of difficult weather conditions. (We had snow and freezing temperatures in late May). Thus the robustness of each hive has been slightly weather dependent, but it appears so far they are not populated with Mites.
Erik is planning to follow up with late summer caging of the queen, followed by another formic vaporization–I will post his results. Note: keep in mind that repeating treatments without rotation, will tend to breed for resistant mites. Better to rotate treatments (such as with thymol). Erik questioned me on this:
A reference from X said that due to the mode of action of OA, it is impossible for mites to gain resistance to it.
The above is a good example of someone talking out of their [hat]. No one even knows for sure what the mode of action of OA is against varroa, nor how it is absorbed. And no matter, I can assure you that some mites will be more resistant than others, which implies that some degree of resistance is possible. Remember, there is only a small margin of safety between the dose that kills mites, and the dose that kills bees. That means that varroa only needs to develop a slight degree of resistance until OA is as toxic to the bees as it is to the mites. Rotate treatments!
My friend Rob Stone (Pierce Beekeeping) recently treated treated a number of packages of bees for sale by spraying a total of 30mL of “weak” solution divided over the four sides of the package cages, and was happy with the results.
Original post
Following the lead of many other countries, EPA has finally approved its legal use for control of varroa. My sons and I have been using oxalic dribble for 15 years with great success. We really like the dribble method due to its low cost, ease of use, safety to the applicator, minimal adverse effects to the colony, and its high efficacy against varroa if applied correctly. Here are some tips:
Application
- The typical dosage of oxalic dribble is 5 mL (1 tsp) per “seam” of bees between the frames. Solution spilled on the top bars doesn’t count. I suggest applying it carefully in order to best distribute it throughout the hive.
- Although some researchers caution about applying more than 50 mL per colony, we routinely treat every seam of bees, even if it takes close to 100 mL total (we may get away with this because our broodless period in the California foothills is very short).
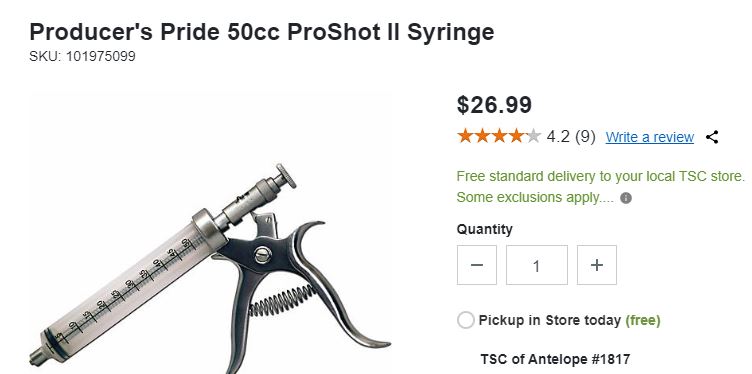
- For application to only a few hives, use a teaspoon or 60 mL syringe (from any feed store).

Dribble being applied with a 60 mL syringe.
- For commercial use, we use a garden sprayer set to a gentle stream, calibrated by the use of a graduated cylinder, to dispense 5 mL per second (1 seam of bees per second, less than 20 seconds per hive).

Calibrating the output of the stream to 5 mL per second. Tip: maintain a large reservoir of air above the liquid–this will reduce the amount of fluctuation in the flow. With practice, it is easy to eyeball the correct stream.
- In fall, we treat the bees in both brood chambers. If the cluster is mainly in the lower box, we tip the upper box back and apply the oxalic from below. If the cluster is mainly in the upper box, we take off the lid and dribble each box from above.

I’m applying the fall treatment while Eric tips back the upper box. We often work in three’s, with two cracking and one squirting.
Treatment windows
- You’ll get the highest efficacy against varroa if oxalic dribble is applied when there is no sealed brood present. This opportunity occurs as a result of natural or induced brood breaks.
- In temperate regions, natural brood breaks typically occur in November through early December.
- Alternatively, you can induce a brood break by making shook swarms, or by caging the queen for 14 days, as shown below.
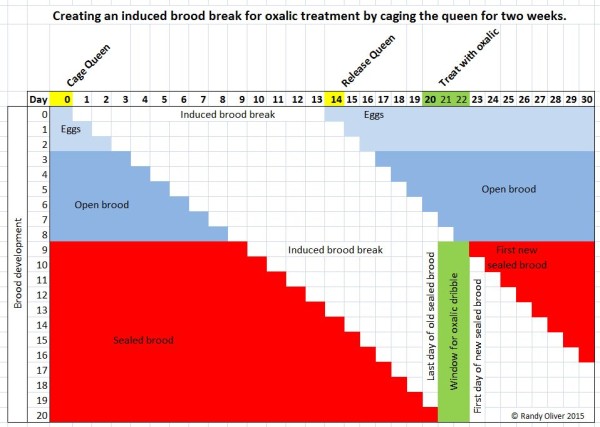
By caging the queen for 14 days, you can create a 2-day window in which there is no sealed brood in which varroa can hide. Note that this window occurs starting about 6 days after you release the queen.
Package bees: Aliano and Ellis, in their very well done preliminary investigation into treating package bees with oxalic, found that the spray application of 3 mL of 2.8% (w:w) of oxalic acid in sugar syrup per 1000 bees resulted in very high varroa kill, with minimal bee kill. Since there are roughly 3500 bees per lb, that works out to:
21 mL of 2.8% OA syrup per 2-lb package,
31.5 mL per 3-lb package, or
42 mL per 4-lb package.
The 2.8% solution is roughly the same as the “weak” formula at Treatment Table. The authors note, however, that their results were preliminary, and I haven’t seen any follow-up research. If you do treat some packages, please let me know the results!
Alternatively, although you could directly treat bees in a package, I’d suggest installing them normally, and then treating them in the hive between Days 5 and 7 after installation. The timing is due to the fact that even if the queen starts laying eggs the day after installation, it wouldn’t be until Day 9 that the first brood would be of suitable age for mite invasion. Oxalic dribble kills mites for roughly 3 days after application. Thus, if you dribble the recently-installed package on Day 6, the full effect of the treatment will have taken place prior to the first opportunity for the mites to hide in the brood.
You can also use this method with shook swarms, or for any divide made without brood.
Nucs: Starting nucs with queen cells in spring presents a great opportunity for controlling varroa by dribbling on Day 19. We’ve now used this method on thousands of nucs, and really like it for getting a “clean start” each spring.
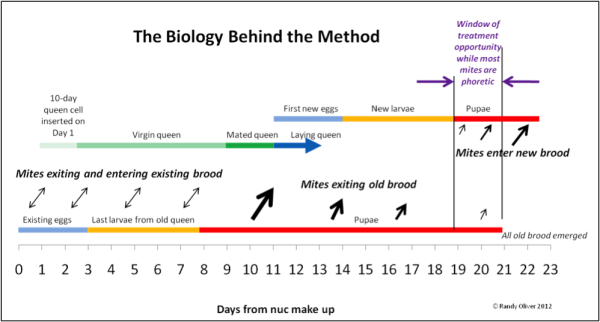
Figure 1. The theory behind the early treatment of nucs—it’s all about timing! There is a brief window of opportunity from Day 19 to Day 21 after make up in which every mite in the nuc is forced out of the safety of the sealed brood. A short-term treatment applied at that precise time could result in a very effective kill of the now-exposed mites!
I’ve fully described this method at https://scientificbeekeeping.com/simple-early-treatment-of-nucs-against-varroa/ .
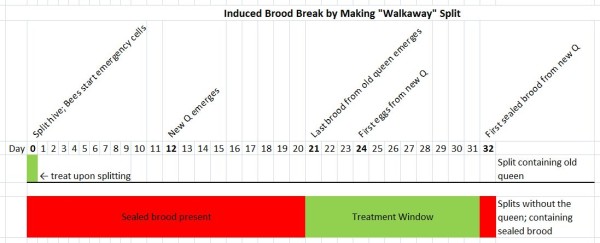
- An even simpler method is to make “walkaway splits”–that is, splitting a hive (into two or more splits) and allowing the queenless split to raise a new queen (although I do not particularly recommend this method, since it depends upon the splits raising emergency queens, plus the splits go without any new brood production for at least 24 days, during which laying workers may develop). The key is to make up the split containing the old queen without any sealed brood (so that all the mites are exposed to treatment). Leave this split on the parent stand to pick up the field force. Into the other (queenless) split(s), place all the sealed brood (any open brood is also fine), along with most of the bees (since all the field bees will fly back to the parent stand). Treat the split with the old queen on the day you make the split(s). Treat the queenless splits on any day from Day 21 through Day 30.
Summer treatment
- Oxalic dribble is not as effective when colonies contain brood (as during spring or summer), but colonies at that time do appear to tolerate stronger or repeated doses due to the rapid turnover of the adult population at that time of year.
- I don’t have data on efficacy, but I’ve treated colonies once a week for three consecutive weeks in late summer without noticing adverse effects (although we prefer thymol or formic acid at that time of year).
Mixing, safety, and storage
- There is a narrow range of dosage that will kill varroa without harming the bees. Follow mixing and application rates meticulously; see https://scientificbeekeeping.com/oxalic-acid-treatment-table/.
- Use common sense when handling oxalic acid crystals. Wear glasses in case of a mishap—you don’t want to get it into your eyes! Wear latex or nitrile gloves to remind you not to rub your eyes.

Weigh the oxalic acid crystals carefully–they cannot be accurately measured by volume (such as by teaspoon measurement).
Note: The wood bleach shown above is not approved for use in bee hives. The only forms of oxalic acid approved for application as a miticide are those registered with the EPA. It is not legal to apply any OA that does not have a label approving it for application for control of varroa.
- Oxalic acid is a relatively strong acid, and is more dangerous to handle than lemon juice or vinegar. You should wear the recommended protective clothing, gloves, and eye protection.
- Based upon my extensive experience with handling OA, wearing safety glasses is the most important, since you definitely don’t want to get it into your eyes!
- The label is the law. That said, I find the recommended glove thickness to be excessive. I’ve found that it’s not big deal when I’ve gotten either the crystals or solution on my skin for a few minutes, and that it can be easily washed off with water. We always keep a jug of neutralizing solution on hand — 10 heaping tablespoons of baking soda dissolved in a gallon of water (the necessary concentration experimentally determined by me). This solution will immediately neutralize any OA (or formic acid) on your skin, protective gear, hive tool, and smoker.
- I do not recommend this, but if I suspect that there is some oxalic syrup on my skin after washing up, I taste my skin with my tongue (OA solution tastes like strong lemonade) just to be sure. This may seem foolhardy, but I can get a dose of up to a full gram of OA in a serving of spinach.
- If your water is hard (contains calcium), use distilled water instead (calcium will cause some of the oxalic acid to precipitate as white calcium oxalate).
- We prefer to first completely dissolve the crystals in hot water, and then add the sugar.
- Oxalic acid in a sugar solution will eventually form HMF [[a]], which is somewhat toxic to bees. It’s unlikely that enough will be formed under normal use to harm the bees, but you should not use a solution that has begun to turn brown. Oxalic syrup can be stored for many months if kept refrigerated [[b]].
[a] Hydroxymethylfurfural, non toxic to humans; commonly found in cooked jams and jellies.
[b] Prandin, L, et al (2001) A scientific note on long-term stability of a home-made oxalic acid water sugar solution for controlling varroosis. Apidologie 32: 451–452. Open access.
First published in: American Bee Journal, June 2013
Part 1: Environmental and Biotic Factors
Setting the Stage
The Lead Up
The Drought
Lack of Good Forage
Varroa
Diseases
Other Indicators of Impending Collapse
An Unexpected Chill
Feedback from Brokers
The Silent Majority
Beekeeper Management
Part 2: The Contribution From Pesticides
The Lynch Mob
Debunking The Myths
The Precautionary Principal
See For Yourself
Be Careful What You Ask For!
The Effect Of Drought
Actions To Take
Bottom Line
References
What Happened To The Bees This Spring?
Part 1: Environmental and Biotic Factors
Randy Oliver
ScientificBeekeeping.com
First published in ABJ June 2013
By now, most everyone has heard that honey bee colonies died in massive numbers this winter. Reporter Dan Rather, in his newscast Buzzkill [1], showed unfortunate beekeepers, some of whom had lost half or more of their colonies, predicting gloom and doom for the bee industry. What were the causes of this year’s bee shortage? As Rather says, “Everyone has an opinion.” The question is whether those opinions are based upon fact! So let’s go over the events leading up to the bee supply debacle.
Setting the Stage
Nearly 800,000 acres of almond trees in California came into bloom this winter—the trees typically start flowering about Valentine’s Day, and the bloom lasts for only about two weeks. Almonds require cross fertilization between adjacent rows of varieties (Fig. 1), and honey bees are trucked in from all over the country to do the job (roughly a million and a half colonies). Many large commercial beekeepers move their hives into California in November to overwinter in holding yards; others build them up on winter pollen flows in Florida or Texas, or hold them in temperature-controlled potato cellars until shortly before bloom. The hives are generally placed into the orchards about a week before the first flowers appear. There is virtually no forage in the orchards prior to, or after bloom in many areas.

Figure 1. An almond orchard in late February, showing the flowering of rows of different cultivars required for cross pollination. The bare “late” varieties have not yet bloomed; the green “early” pollenizers have finished bloom. Grading of colonies is normally done during the bloom of the main crop (usually Nonpareil).
The Lead Up
Two seasons ago there was also a shortage of bees in almonds, following the coldest January (2011) in 17 years (cold being a major stressor of wintering bee colonies). Beekeepers then replaced their deadouts with package bees and splits, thus starting a new generation of colonies, which tend to have lower varroa mite levels than established colonies. These colonies entered autumn 2011 in pretty good shape, and then enjoyed the fourth warmest January (2012) on record! As a result, there was the lowest rate of winter mortality in years, and plenty of bees for almonds in 2012 (Fig. 2).
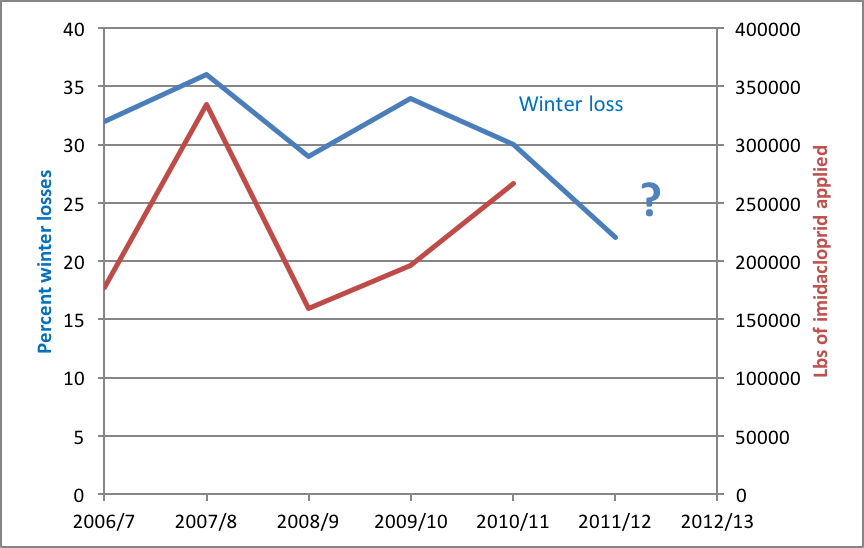
Figure 2. Percent winter losses since the beginning of the national survey—the data is not yet in for 2012/13. Note that there has been a general downward trend, suggesting that whatever caused the high losses in 2007/8 has not been such a problem in recent years. Note also the cyclical nature of colony winter losses, with high losses in 2004/5, 2007/8, 2009/10, and 2012/13 (some data not shown) Data from [[i]].
[i] http://www.ars.usda.gov/is/pr/2012/120531.htm and California DPR.
I was curious as to whether the colony loss rate was linked to the use of neonicotinoid insecticides. There is no recent USDA data, so I went through the California Pesticide Use Reports (data available through 2010). I plotted the amount of imidacloprid applied to crops in California in the preceding year in red (the seed treatment clothianidin didn’t even make the top 100 list of pesticides applied). Although there appears to be a possible correlation from 2006 through 2009, the trends were reversed for 2010. I will be curious to add the 2011 data when it becomes available.
In March of 2012 I received a phone call from a California queen producer who had a prescient insight as to a potential brewing disaster. He was receiving calls for queen bees from Northern beekeepers whose bees had already grown to swarming condition due to the unseasonably warm spring weather (Fig. 3).
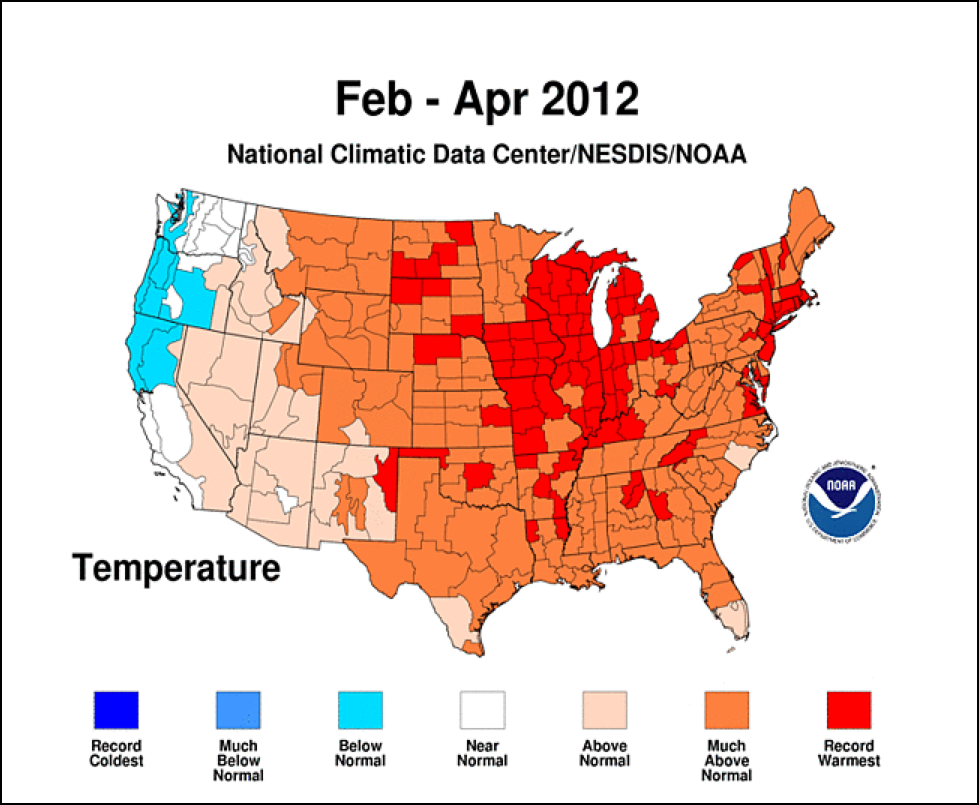
Figure 3. Last year’s warm spring in much of the country lead to early broodrearing, and as a result, early buildup of varroa levels. Note the record warm spring in the Midwest.
The queen producer noted that such early brood rearing also meant early mite buildup, and predicted that since most Midwestern beekeepers treat for mites by the calendar, that they would unknowingly allow mites to build to excessive levels before treatment. This was strike one against the bees.
The Drought
Then it didn’t rain–by midsummer, it was clear that the continental U.S. was in serious drought, including California, whose beekeepers supply nearly half the bees for almond pollination. The only ways that we kept our colonies strong was to either feed expensive pollen supplement and sugar syrup, or to move them to elusive better pasture out of state. By late summer, 60% of the U.S. was in drought, meaning that unless your bees were next to soybeans or irrigated crops, there was little forage for them. This lack of good nutrition was strike two against the bees (Fig. 4).
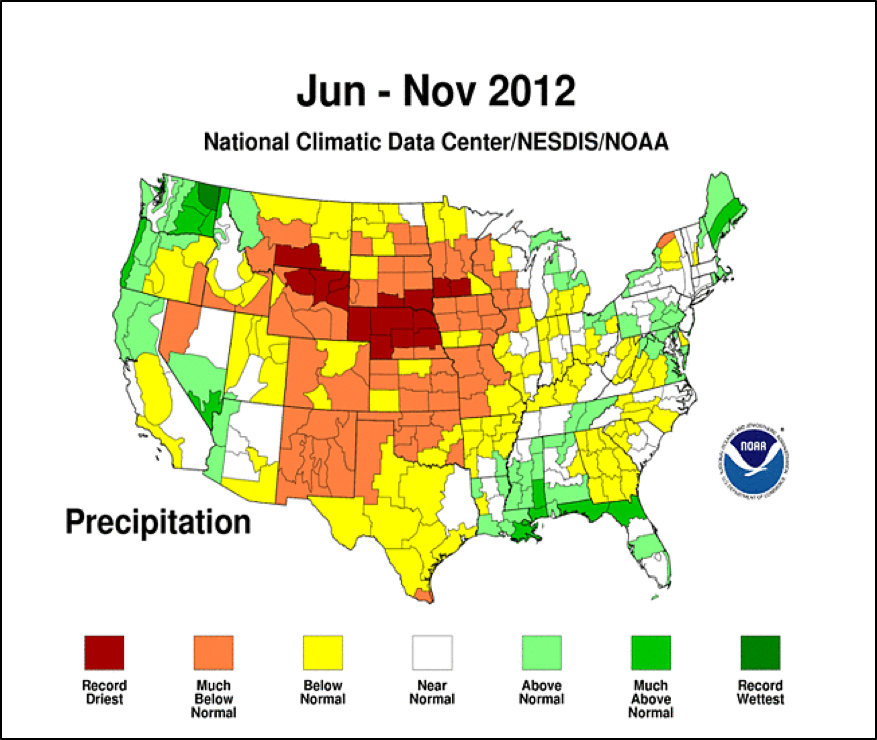
Figure 4. The severe drought in the Midwest really put the hurt to bee pasture in those states in which the majority of commercial hives spend the summer. Source [[i]].
[i] http://www.ncdc.noaa.gov/temp-and-precip/maps.php
Drought not only dries up nectar and pollen sources, but also forces bees to fly further and more frequently for water. Plus it concentrates ag chemicals and pesticides in the few sources of surface water available to bees. The bees started to show the hurt.
Beekeepers tried to move their hives to areas of better forage, sometimes overstocking an area with too many hives, which led to excessive competition for resources, and the spreading of parasites. Others desperately chased less desirable crops such as sunflowers. Colonies in holding yards in California found little to eat, due to our record dry weather. Some beekeepers with winter eucalyptus locations found them crowded with other hives.
Lack of Good Forage
In Buzzkill, Bret Adee brought up the fact that bee pasture in the Midwest is disappearing under the plow, largely due to our environmentally-irresponsible taxpayer-subsidized policies that encourage farmers to plant every square foot of land into corn (Fig. 5). Bee brokers told me that colonies coming to almonds from the Midwest were in generally poorer shape this year than those coming from the southern states.
Practical application: some Midwestern beekeepers split their operations, hauling some to the South to rebuild over winter, and the rest directly to California–there was a night and day difference as to how the colonies looked in February!
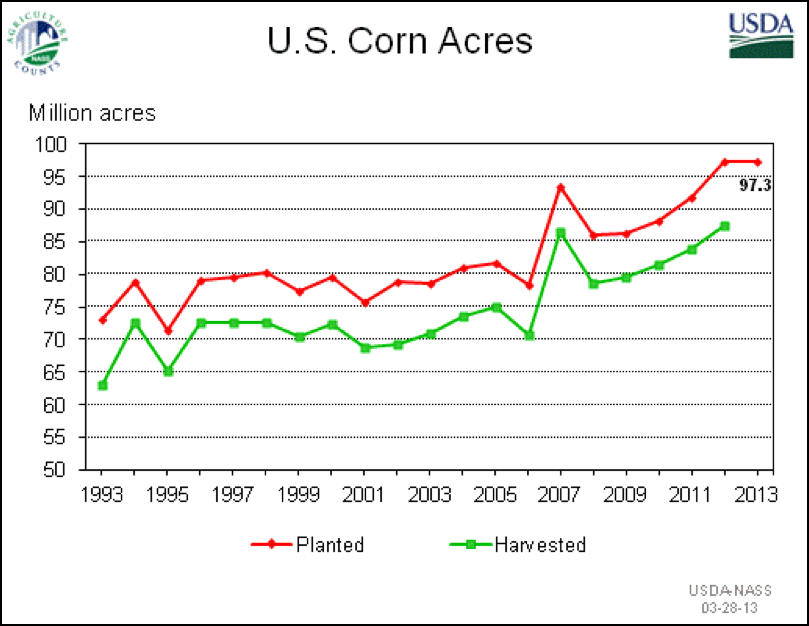
Figure 5. Grasslands and wetlands in the Corn Belt are rapidly being converted to monocultural, heavily herbicided corn/soy, which eliminates virtually all bee and wildlife forage. A new study found that between 2006 and 2011 there was a net loss of 1.3 million acres of grassland. This affects not only bees—the authors [[i]] state that “As a consequence, populations of grassland nesting birds are declining faster than any other group of birds in North America.”
[i] Wright, CK & MC Wimberly (2013) Recent land use change in the Western Corn Belt threatens grasslands and wetlands. https://www.motherjones.com/files/pnas201215404_nwow5w1.pdf
To put this loss of bee pasture into perspective, I asked some Dakota beekeepers for estimates of how many acres of CRP grassland are needed to sustain a colony of bees. In recent years, the overall hive density in North Dakota has been more than 10 hives per square mile (less than 64 acres per hive, including wastelands).
Practical application: the best guess by those beekeepers was that each colony of bees requires about 5-15 acres of productive land for forage (late summer forage being the critical factor). If we use the figure of 10 acres per colony, then the conversion of 1.3 million acres of grassland to herbicided cropland suggests that forage for 130,000 colonies of bees has been eliminated in the past five years in the Corn Belt alone! This figure represents nearly 9% of all colonies needed for almond pollination.
Varroa
An excellent window into the causes of colony health problems is the USDA National Honey Bee Pests and Diseases Survey Report [5] (the latest data have not yet been released). It is worrisome that varroa levels appear to be steadily climbing year after year.
And if the drought and forage problems weren’t enough, the favored miticide of commercial beekeepers became unavailable for a time last summer, and mite levels built to killing levels in a number of operations. By late July, some of us were already predicting a disaster for the upcoming almond pollination season. Although many beekeepers finally got mite levels down with late-season treatments, the damage had already been done, and there was no turning the colonies around. Strike three for the bees!
In November semi loads of hives started moving into California, or had been placed in potato cellars. Some of the colonies that arrived from the Midwest were in poor shape, or crawling with mites. Oddly, few beekeepers at the time owned up to having problems, despite the reports that I kept hearing of mite and forage issues! I’m not sure whether this was due to denial, wishful thinking, simple lack of lifting the lids, or something else.
Diseases
Nosema infection also runs rampant across the country—70% of colonies were infected in June of last year. The stressful factors leading up to almond bloom apparently put a lot of hives close to the “tip point” at which pathogens can overwhelm the colony immune system and start it going backwards, or initiate the slide into sudden depopulation (detailed at [6]). Few seem to be mentioning signs of CCD–it is unfortunate that the media keep using that term as a catch-all for all hive problems!
One should keep in mind that the winter collapse issue appears to be cyclical, similar to flu or other pathogen epidemics. I have strong reason to suspect that the constantly-evolving viruses are involved in these colony collapse epidemics.
There has also been a strong resurgence of European Foulbrood and other unidentified brood diseases [7] (Figs. 6, 7, and 8). Unlike EFB of old, the new forms don’t go away with a nectar flow.

Figure 6. “Shot brood” due to EFB. Note the fat queen near the center. Despite her vigorous egglaying, this colony is unable to pull ahead due to excessive brood mortality. Lots of beekeepers reported EFB symptoms this winter.

Figure 7. You really have to look hard in some colonies with spotty brood to see the cause! Two larvae in this photo show signs of EFB infection.

Figure 8. Dying brood from one of my sick colonies this spring with EFB-like symptoms. Note the “shot” pattern, the twisted larvae, and the dried larval remains. There is also some AFB-like coloration, but lack of roping or AFB odor (this odor is distinct and sour), nor a positive Holst milk test. In this colony, even pupae were dying. I observe these symptoms independent of whether the hives went to almond pollination or not. Colonies with this (or similar) infection cannot grow. Treatment with oxytetracycline generally clears it up.
One thing that I noticed in Buzzkill was the uneaten pollen supplement patties in many of the crashed hives. I’ve mentioned before [8] that I’ve found a colony’s failure to consume pollen supplement to be a reliable predictor that that colony will later collapse.
Another strong predictor of winter collapse is weak strength in fall (upcoming article), again strongly suggesting that those colonies already have some sort of health issue going into winter. I heard reports from all over the country that bees went into winter in poor condition.
An Unexpected Chill
The final blow to hives in California was a blast of icy weather (Fig. 9). This unexpected chilling compounded all the existing problems! I’ve previously pointed out that colony collapse often follows unseasonable chills, since it shifts the tip point for virus and nosema epidemics. Clusters that had expanded for broodrearing contracted, resulting in chilled brood and dead young bees on the ground. My own colonies simply shut down broodrearing completely, losing about two weeks of buildup.
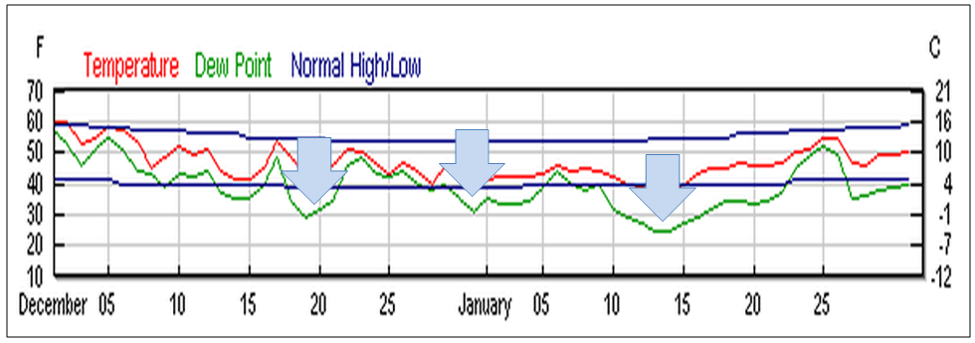
Figure 9. Chilling events (blue arrows) in Modesto, California this winter (the dark blue lines represent normal highs and lows). The unusual chilling in late December and early January (hitting the ‘20’s in a number of areas) came at the time when colonies normally begin to build up for almonds. This severe (for California) cold set the already-stressed colonies back hard, and may have allowed nosema and viruses to gain the upper hand. Graph from wunderground.com.
At the national convention in January, the first reports of beekeepers with collapsing operations were heard. But still, the industry was in denial, with an apparent glut of promised bees as late as the end of the month (two weeks before start of bloom)! But when the rubber finally hit the road in mid February, that illusory supply quickly evaporated, with desperate growers and brokers scrambling to obtain bees—some offering obscenely high prices for substandard colonies.
And then, due to the cool spring, the trees held off on blooming for an extra 10-14 days [9]–colonies placed in anticipation of normal start of bloom just sat there starving and shivering on the cold orchard floors.
Practical application: the biology here is that this is the time of the “spring turnover” in bee populations in California, during which the old overwintered adult bees must rear their replacements for the spring buildup of population. The conditions in the almond orchards prior to bloom are miserable for smaller colonies—it is warm enough to encourage them to break winter cluster and expand the broodnest, but overnight frosts on the Valley floor can cause serious chill stress. Furthermore, it is often warm enough to fly at midday, but there is virtually nothing to forage upon until the trees start blooming! Such fruitless foraging further wears out the workers, and allows sick bees to drift to adjacent hives. Worse yet, the desperate foragers rob out any dead or dying colonies in adjacent orchards, rapidly and effectively transmitting mites, nosema, viruses, and anything else harmful in the deadouts.
Many colonies went backwards during this excruciatingly long wait. Some beekeepers told me that hives graded at placement scored better than those graded at bloom (just the opposite of normal)!
I’ve been carefully observing spring turnover in my “dinks” (weak colonies) in February (Fig. 10). What I find is that the problem is generally not the queen; rather, the colonies are infected with some pathogen– most commonly nosema [10], the paralytic viruses [11], or EFB (or EFB-like brood disease). Those colonies that are able to successfully emerge one solid round of brood are often able to “clear” the infection and completely rebound by April. Those that get hit by frost in February often collapse.

Figure 10. An example of an unsuccessful spring turnover. This colony is in the middle of typical February collapse from nosema or IAPV. You can easily see the outline of the area recently covered with brood, delineated by the crescents of freshly-packed pollen. Colonies undergoing this sort of depopulation tend not to forage for nectar, and do not respond well to supplemental feeding. This colony continued to collapse quickly, and finally died in a cold snap a week later—with only silver-dollar sized patch of dead bees remaining.
Feedback From Brokers
I asked a few of the major pollination brokers for their observations on the colony shortage this season. Their feedback suggested that the causes for the bee shortage were varied and many.
Summary:
- Most were able to eventually fill their contracts. Beekeepers often hold colonies in reserve “just in case,” or gambling that in “short” years they can rent those last hives at an elevated price. Also, when the offered price went up, hives not originally intended to go to almonds were loaded up at the last minute and shipped to California (I was in Florida at the start of bloom, and had an inspector tell me of certifying colonies for shipment after the bloom had already begun!).
- A number of hives received in November were already headed downhill. Some exhibited the symptom of bees not clustering properly (a typical sign preceding sudden colony depopulation/CCD). Some arrived crawling with mites, or with recent mite treatments in place (suggesting that they were treated too late).
- Some graders saw piles of dead bees in front of hives—cause unknown. There were reports of some herbicide tank mixes killing bees.
- Many of the placed colonies were below standard grade— growers paid for less than they expected!
- Graders told me that there was a huge variation in hive strength from beekeeper to beekeeper. Many hives were strong (12-16 frames of bees) and healthy; other operations graded at zero to three frames of bees (some of the deadouts had spider webs inside, suggesting that they hadn’t been occupied by bees for some time).
- The unusual winter chill was tough on colonies that had been stimulated into early buildup, and then forced to contract their broodnests. Some colonies kicked out chilled brood and dead bees afterwards.
- Many beekeepers watched their colonies go “backward” prior to bloom.
- Colonies from the Southern states (especially those delivered in February) were generally in better shape than those from the Midwest.
- Midwestern beekeepers blamed drought, mites, poor nutrition.
- Several beekeepers said that their best bees came from remote areas, and their worst from ag areas.
- A number of beekeepers admitted inadequate mite treatment; mites were a recurrent theme.
- There were a number of reports of EFB hitting colonies.
- Some had gotten hit last summer with pesticide sprays, and their colonies didn’t recover.
- “There were good bees and bad bees from every state. They all seemed to have different problems depending on location/state.”
- Many good beekeepers simply didn’t know what happened to their hives; there were lots of lifeless hives delivered. The atmosphere was ripe with speculation as to the actual causes.
- “The shortage was also created by beekeepers that chose not to come to California for a variety of reasons. They can make more money with honey, didn’t get paid for what they have brought in the past, bees come back home with mites, beetles and whatever else takes a ride on the hives. Beekeepers don’t want to risk bee health to chase the dollar.” Many out-of-state beekeepers have had bad experiences going to almonds, and simply don’t feel that it’s worth it. The supply of bees will largely depend upon the price that growers offer for renting them!
The Silent Majority
Buzzkill leaves one with the impression that the entire bee and almond industries are on the verge of collapse. Of course, the news media focus on fear and disaster, so we may consider taking such dire projections with a grain of salt. In the case of Dan Rather, the focus was on the beekeepers with troubles, not upon those who successfully filled their pollination contracts.
So just how severe was the problem? Let’s say that there was an overall shortage of 100,000 hives (a figure that I heard floated)—that would represent only about 6% of the total number of hives placed into almond pollination. The other 94% were successfully delivered (although a proportion of those were weak due to the poor season).
Since the debacle, I’ve heard from plenty of beekeepers whom I’ll refer to as the “silent majority,” who experienced “normal” colony winter losses in the 5-25% range, and who successfully filled their pollination contracts. Although the hearts of all beekeepers go out to those who suffered severe colony losses, many felt that some of those losses could have been prevented if the afflicted beekeepers had been more proactive than reactive.
And don’t forget those upon whom the rest of the industry depends to supply bees for restocking their deadouts! The California package producers, who have been pollinating almonds for decades, are routinely counted on to consistently take strong hives to almonds, and to then shake over a hundred thousand packages of bees for sale afterwards. Few of these major producers experience severe unexplained colony losses.
Beekeeper Management
By no means am I suggesting that those beekeepers who suffered losses engaged in poor beekeeping practices, but I can’t help but notice that not all beekeepers were equally affected—a great number provided strong, healthy colonies to almonds. I’ve spoken to some of them–the common thread is that those who recognized the problems of poor nutrition and mites in August, and took remedial action for the rest of the season, had acceptable winter losses.
Some beekeepers who really put serious effort and money into bee husbandry were even able to sell “shook bees” from their colonies to others in February! For example, watch Keith Jarrett feeding substantial quantities of pollen supplement to very strong colonies in January [12]—Keith consistently brings very strong colonies to almonds every year, and this year was no exception!
Practical application: I’m here to tell you, that one lesson that I’ve learned during our intense California drought, is that those yards that I fed with protein in late summer before they started going downhill went to almonds much stronger than those that I didn’t feed until fall! Proactive is better than reactive—if you wait until colonies are already going downhill, it is much more difficult to turn them around!
I’ve often been accused of being politically incorrect for speaking frankly. I’d like to make amends at this point by retiring the rude and unsympathetic term “PPB” (Piss Poor Beekeeping). The fact is that the average wintering loss for the past few years has hovered around 30%. So if you experience 30% losses, you can now proudly call yourself an “Average” beekeeper!
But what about those beekeepers who consistently manage to enjoy lower rates of winter loss? I propose that we call them “Lucky” beekeepers, and the best of them, “Consistently Lucky.”
Practical application: the harder those beekeepers work, the luckier they get!
But there were clearly “unlucky” beekeepers this year—especially the “big boys” who brought tens of thousands of hives from the drought-ravaged, and corn-converted Midwest to California. California beekeepers are used to summer drought. We have learned to either move our colonies to better (often irrigated) pasture, or to feed expensive pollen supplements. This would be a very expensive proposition to the larger operators, with hives spread all over the place—a cost not covered by current pollination prices.
What Happened To The Bees This Spring?
Part 2: The Contribution From Pesticides
Randy Oliver
ScientificBeekeeping.com
First published in: American Bee Journal, July 2013
It’s pretty straightforward to attribute the majority of colony losses this winter to the usual and aforementioned causes, but a number of beekeepers are also pointing the finger at pesticides. There is no doubt that in certain areas pesticides were a serious issue to beekeepers. Colonies set back by pesticide kills may not fully recover over the season, and those going into winter with pesticide residues may go downhill. There is also reason to suspect that pesticides and miticides have something to do with today’s high rates of queen failure.
The bees in some drought-stricken areas were forced to forage on irrigated and pesticide-laden crops—the only place in which there was anything to eat. This changes the entire dynamics of pesticide exposure, since residues would no longer be diluted by the pollen and nectar of non crop plants. The lack of good natural forage also suppresses the ability of colonies to deal with the insult of those pesticides. And colonies may be forced, by necessity, to forage upon one treated crop after another, resulting in multiple exposures.
Practical application: under drought conditions, bees may suffer more from pesticides than when times are good.
Due to the current high prices for agricultural commodities, farmers are often applying pesticides indiscriminately as “risk insurance” rather than due to actual need. A chilling recommendation from an extension entomologist reads:
I encourage you to be risk averse and to make an investment that will pay dividends for your valuable crop. Consider applying [flubendiamide, indoxacarb, or spinosad] for corn earworm. If you have stink bugs and are in the [mature plant] stages, you might want to tank mix one of these products with a pyrethroid. A tank mix of a pyrethroid and acephate are an option, but will wipe out all beneficials [13].
The first three insecticides mentioned are considered to be “reduced risk” to bees if residues are allowed to dry for a few hours, but no mention was made to spray at night. Of the five insecticides recommended above for spraying on corn in tassel, at least four are highly toxic to bees if sprayed during the day! No farmer wants to kill bees, but with recommendations like this from state extension agents, well-meaning growers may unwittingly be hurting pollinators.
Bees in agricultural areas are exposed to a vast array of insecticides, miticides, fungicides and surfactants—many of which have clear links to colony health problems. And applications of new mixes of chemicals are up. For example, in addition to the neonicotinoid seed treatments, granular insecticide soil treatments for corn in the Midwest were up by 30% over the previous year [14]. These treatments consist of combinations of organophosphates and pyrethroids.
But I’m not hearing either the bird groups or beekeepers even addressing these treatments! It is scary to read the sales literature for Counter insecticide, the organophosphate terbufos [15]. Growers are encouraged to apply it at planting time, despite the facts that:
- “Terbufos is highly toxic to birds, fish, and aquatic invertebrates [and bees]. [It] shows significant acute mortalities of birds, mammals, reptiles, and fish resulting from broadcast application…In the same study, the application of terbufos as a soil-incorporated treatment to corn…resulted in acute mortalities to birds and reptiles” [16].
- Terbufos is strongly systemic, meaning that it is absorbed by the plant roots and could be expected to be expressed in the pollen and nectar.
- It can synergize with other pesticides since it ties up the critical CP450 enzymes used in detoxification, to the extent that growers are cautioned that it can cause problems to corn from herbicides [17].
During drought, certain insect pests become more problematic, perhaps resulting in increased exposure to insecticides by bees. For example, drought encourages corn leaf aphids. Read this chilling recommendation for aphids on corn during tasseling (when bees are actively foraging):
If less than 50% of pollination has occurred, aphids and honeydew are covering tassels and plants are stressed, an insecticide may be necessary to ensure adequate pollination, but treatments need to be made within 48 hours of tassel emergence. Asana XL, Brigade, Capture, Cobalt, Dimethoate, Lannate, Lorsban, or Malathion may be used for control [18].
Or this:
Prolonged drought always raises the specter of two-spotted spider mite outbreaks in soybeans and corn. As the 2012 drought intensifies in Minnesota, infestations are reaching treatable levels…The only products that are recommended for spider mites in soybean include insecticides containing chlorpyrifos, dimethoate and bifenthrin[18].
The names of the recommended insecticides above strike fear into the hearts of beekeepers!
Practical application: many “consistently lucky” beekeepers go to great effort to allow their colonies to recover after exposure to pesticides—moving them to unsprayed areas or natural forage, or by immediately feeding protein supplement to stimulate increased broodrearing. Unfortunately, such “recovery” areas are getting harder and harder to find.
The Lynch Mob
Despite the fact that a wide range of bee-toxic insecticides are being applied (often during bloom) to corn, soy, sunflowers, alfalfa, cotton, and other major crops, if you Google anything about insecticide use, you’ll quickly find that the blogosphere focuses only upon the putative link between a single class of insecticides—the neonicotinoids–and the demise of pollinators [19].
People look at me incredulously when I point out that there is zero firm evidence to date that the neonic seed treatments are a serious problem! But the notion that all honey bee problems are caused by an insidious new insecticide resonates with a distrustful public [20], and has firmly established itself as “common knowledge.” But repeating something does not make it true!
“It’s easier to fool people than to convince them that they have been fooled”–Mark Twain
Practical application: the question is, “Are the neonic seed treatments being railroaded into a guilty verdict in the media’s kangaroo court of public opinion?”
One group recently brought suit against the EPA to ban the use of the seed treatments clothianidin and thiamethoxam [21], neither of which even make California’s top 100 list of pesticides applied [22], nor that have ever been demonstrated to harm colonies feeding on the pollen or nectar of seed-treated plants! A number of people have made up their minds that the neonics are the main cause of colony collapse, and it appears that no amount of facts to the contrary will cause them to reconsider!
Debunking The Myths
As anyone who knows me will tell you, I am a stickler for honesty, accuracy, and factuality. I am concerned about the amount of misinformation and speculation going around about the neonics. So let’s look at some of the claims vs. the actual facts.
| Arguments Against Neonic Seed Treatments |
Actual Facts
|
| The neonicotinoids have been “linked” to increased colony mortality. |
In actuality, such a “link” is merely an urban legend, and has never been demonstrated or confirmed in any study.
On the other hand, the residues of other classes of pesticides are more suspect for causing increased brood or adult bee mortality [24]. |
| The timing of CCD coincides with the introduction of the neonic seed treatments in 2004. |
CCD started in California bees in the winter of 2004/2005, prior to them ever being exposed to seed-treated crops. |
| But what else could have changed at that time other than the introduction of neonics? |
In California, Dr. Eric Mussen [25] determined that the increased colony losses were due to poor summer forage and failure of mite control products (just as this last winter).
There is actually a much stronger association between the incidence of the novel gut parasite Nosema ceranae and increased colony mortality [26].
But the main thing that has changed is the dynamics of the varroa/virus complex, which coincidentally occurred at about the same time that the neonics came into use. |
| European countries banned the neonics, and the bees recovered after those bans. |
A few countries placed temporary suspensions on certain seed treatments until planting dust issues were resolved [27]—only Germany has one suspension still in place. The foliar applications were not suspended. The suspensions did not resolve bee health problems. |
| The European Food Safety Authority recently decided that neonics pose a threat to bees. |
“The Center for Regulatory Effectiveness (CRE) has recently completed a Data Quality Act (DQA) Alert on the … (EFSA) report on neonicotinoids which found that neonicotinoids pose a risk to bees. The DQA Alert outlines the serious deficiencies of the EFSA report and demonstrates why the EFSA report violates the DQA…In particular, the EFSA report failed to maximize the objectivity of the data by failing to reconcile numerous studies whose conclusions contradicted the findings of the EFSA report” [28]. |
| Several lab studies have found that neonics affect individual bee behavior, longevity, or immunity. |
True — although many studies used unrealistically high doses. The question is whether such artificial studies apply to actual colonies in the field. The numerous field studies to date have failed to find any link between seed treatments and later colony health issues. |
| It is the seed treatments that make corn a problem. |
As Bret Adee points out in Buzzkill, corn is replacing pastureland (Fig. 4). Corn, as grown today, is a virtual “bee desert” (similar to the way in which suburban lawns are green bee deserts). And it’s not only the bees that this is affecting, the populations of birds and other wildlife are plummeting due to loss of favorable habitat (see my blog on birds and neonics [29]).
A recent survey by Dr. Jerry Bromenshenk found that bees actually avoid field corn pollen, and are exposed to very little of the seed treatment residues [30].
Numerous independent studies, and the experiences of stationary beekeepers throughout the Corn Belt, support the conclusion that colonies can thrive when surrounded by corn, provided that there is some alternative forage within flight range. |
| As the use of neonic seed treatments increases, bee mortality goes up. |
In actuality, colony mortality rates go up and down year to year, largely dependent upon weather and varroa mite control. If the neonics were to blame for this winter’s bee losses, why didn’t they cause similar losses last winter, in which the colony mortality rate was the lowest in years? |
| French beekeepers also started seeing problems with the introduction of the neonics. |
I’ve spoken with beekeepers in France whose apiaries are in pesticide-free areas. They tell me that they experience the same sorts of colony mortality problems as do those in areas exposed to neonics. |
| Bees in the U.S. are commonly exposed to neonicotinoids. |
In the most recent USDA survey (100 samples across the country), imidacloprid was only detected in 9% of the samples [31] (although I found some of the residue levels alarmingly high). However, the most common seed treatment, clothianidin (or its degradation products), was not detected at all!
The above real-world data suggests that efforts to ban clothianidin as a seed treatment may be misplaced. It appears that imidacloprid, especially as a foliar application, would be of more concern. |
| Neonics are the most common pesticides that bees are exposed to. |
In the above survey, other serious insecticides were more commonly prevalent: chlorpyrifos (in 20% of samples), cyhalothrin (in 7%), and endosulfan (in 11%).
Notably, there was also a high prevalence of beekeeper-applied miticides: fluvalinate (in 38%), coumaphos (in 87%), amitraz (in 27%), fenpyroximate (in 11%), and thymol (in 27%).
There was even higher exposure to fungicides and adjuvants. |
| It is misleading for the pesticide companies to blame the problems on varroa, nosema, or poor nutrition. |
The above survey (over 1000 samples) found that the average varroa infestation rate in the U.S. in autumn is above the danger level for virus epidemics!
Sixty to 100% of hives are infected with nosema in December.
Summer drought has historically been associated with high winter mortality. |
| But didn’t the planting dust from corn seeding kill colonies in Ontario? |
Planting dust is separate issue that clearly needs to be remedied. It does on occasion cause bee kills, for which beekeepers are rarely compensated. This situation must change! All parties are actively working on solutions [32]. |
| Bees in certain agricultural areas tend to go downhill later in the season. |
This has been observed for a long time—long before the neonics. The question is, which chemicals, chemical synergies, or chemical/nutrient interactions are responsible? The Frazier/Mullin team at Penn State has developed a protocol for helping to figure this out. I strongly support its adoption by the EPA for pesticide risk analysis. |
| Colonies foraging upon nectar or pollen of seed-treated crops get poisoned. |
Ask yourself this: if neonic residues were actually so harmful to bees, how is it that the Canadian beekeepers, whose bees forage largely on seed-treated canola, feeding solely upon a diet of canola nectar and pollen with well-documented residues of clothianidin, experience very low winter losses, despite the long Canadian winter (so long as they control varroa and nosema)?
And how is it that the vast majority of beekeepers in the U.S. Corn Belt report that their colonies thrive and that they have far fewer pesticide issues these days than in the past? |
| The neonicotinoids are “systemic,” meaning that they are in the plants all the time! |
True, but this property is not unique to the neonics—a number of other insecticides also go systemic. In any case, with seed treatment, the concentration of the insecticide in the plant is only high when the plant is young—it gets diluted as the plant grows (e.g., clothianidin in canola is at a level high enough to kill aphids for only about the first 30 days of growth).
The only time that residues in the plant matter to pollinators is when the mature plant flowers. The amount of seed treatment is carefully calibrated so that the residue in the pollen and nectar are below the level that causes demonstrable harm to bees.
In the case of foliar, drench, or chemigation applications prior to bloom, there are greater possibilities for bees to be exposed to toxic levels. |
| There are fewer butterflies and pollinators in the fields these days. |
Not surprising, since the new push for “clean farming” has removed the host plants upon which the butterfly larvae feed. Pollinators are forced to subsist upon the stretches of weeds growing along roads at the edges of fields. But surprisingly, pollinators may be abundant there, suggesting that even though populations as a whole are reduced by habitat conversion, it is that, rather than the use of seed treatments, that causes the population declines. |
| The evil pesticide companies want to kill honey bees. |
Give me a break! Does anyone truly believe that anyone wants to kill honey bees? What pesticide company would want the bad press of being associated with killing bees? The chemists and biologists on their staffs earnestly work to develop insecticides that are bee friendly. |
| The EPA is being derelict in their duty to protect pollinators. |
I have spoken at length with EPA staff, and reviewed their risk assessments, as well as those by, DEFRA, EFSA, PMRA, and other regulatory agencies. I find that the risk assessors have not overlooked any evidence, are well-informed on the subject of neonics, and are justified in their assessments that the on-the-ground evidence (to date) indicates that neonic seed treatments pose acceptable risk to pollinators. |
| We must all remember that the tobacco industry tried to hide the fact that nicotine was addictive [33]. |
Spare me! Does anyone seriously think that the EPA is unaware that industry executives may stretch the truth? Of course the EPA is skeptical of any reassuring claims by the pesticide industry—that’s why they go over all studies with a fine-toothed comb! |
| This winter’s losses spell the end to commercial beekeeping. |
The fact of the matter is that many observers note that the bee supply for almonds often follows a boom-bust cycle. Although losses were high this year, the trend for the last decade has been for beekeepers keep ramping up the supply of bees for almonds. So long as growers are willing to pay a profitable rental rate for colonies, market forces will encourage the bee industry to meet the demand (for a detailed analysis, see [34]). |
The Precautionary Principal
“But,” you say, “shouldn’t we exercise precaution due to the lab studies that find adverse effects from the neonics?” Look, I make my living as a beekeeper, I’m not out to sell insecticides, and am as concerned as the next person about the environment and the safety of the food I eat. I’ve researched the neonics exhaustively, and addressed them in several articles [35]. I am acutely aware that there are suggestions that the neonics may be causing insidious effects in the environment, and I’ve studied the excellent environmental document Late Lessons from Early Warnings [36], which hammers the message that we should use the “precautionary principle” when dealing with chemicals. The problem is, there is nothing without risk—for example, you have a 1 in 83 chance of being killed in an auto accident in your lifetime. But most people still take the risk of getting into cars, since they feel that the benefit outweighs the clearly high risk!
My practical perspective as both a scientist and a beekeeper: if researchers perform lab studies on any insecticide, they will find that there are all kinds of negative effects upon bees—this should be pretty obvious, since insecticides are specifically designed to harm insects! However, the majority of these studies are taken out of the context of full colonies under field conditions, where bees fly free and choose the flowers upon which they forage. The evidence to date supports the contention that the neonics, properly used as seed treatments, are indeed an improvement over other insecticide options.
As Dr. Eric Mussen succinctly notes:
Nobody’s really been able to show that [the neonicotinoids] are more problematic than the rest [of the pesticides to which bees are exposed] [37].
Far be it from me to suggest that the neonics (or any other pesticides) are harmless! But consider this—if the neonic seed treatments were indeed as harmful as some make them out to be, you’d think that after a decade of intense study that at least one researcher could have come up with a single solid piece of field evidence against them!
Let’s do a thought experiment. Why doesn’t someone simply put a bunch of healthy hives into the middle of seed treated crops and see whether they die afterward? Oh, I forgot—this experiment has already been run by thousands of beekeepers year after year in the Corn Belt and the Canadian prairie! And those beekeepers have invited me to look at their colonies, sent me photos of colonies stacked head high with honey supers, and bragged about their high winter survival!
Some will argue ’til they’re blue in the face, but the fact remains that virtually every beekeeper that I’ve spoken with in the Corn Belt and in canola areas feels that the seed treatments are not a problem [38]. In fact, most tell me that this is the best it’s ever been as far as bees and pesticides!
Common sense: I just don’t get what is so hard to understand about the reality that there are thousands of colonies thriving year after year in areas of intense seed treatment? To any reasonable person it would suggest that the treatments are causing little noticeable harm other than the occasional planting dust kill, which I have repeatedly stated is a problem that needs to be corrected!
See For Yourself
Let’s look at actual independent (from the manufacturer) data from corn and canola areas:
Corn
I asked friends in the Corn Belt if they had any data on winter losses. It so happens that the Michiana Beekeepers Association has been collecting exactly that since the spring of 2010 (Fig. 11).
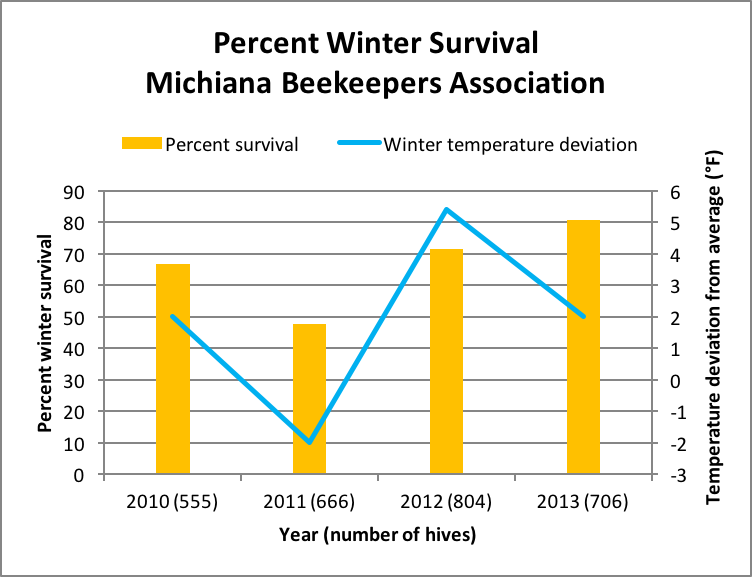
Figure 11. Percentage of winter losses by the “Michiana” hobby beekeepers. The 2013 figure is as of mid March; it may eventually go down a bit due to a prolonged cold spring. Note that the winter survival rate appears to be linked to average winter temperature. Thanks to beekeeper Danny Slabaugh for sharing the data; temp deviations from [[i]].
[i] http://www.ncdc.noaa.gov/temp-and-precip/maps
How could the above be? Eighty percent winter survival despite sitting in the middle of seed-treated corn and soy? So of course I did a fact check to confirm that those beekeepers were indeed sitting in corn/soy areas (Fig. 12).
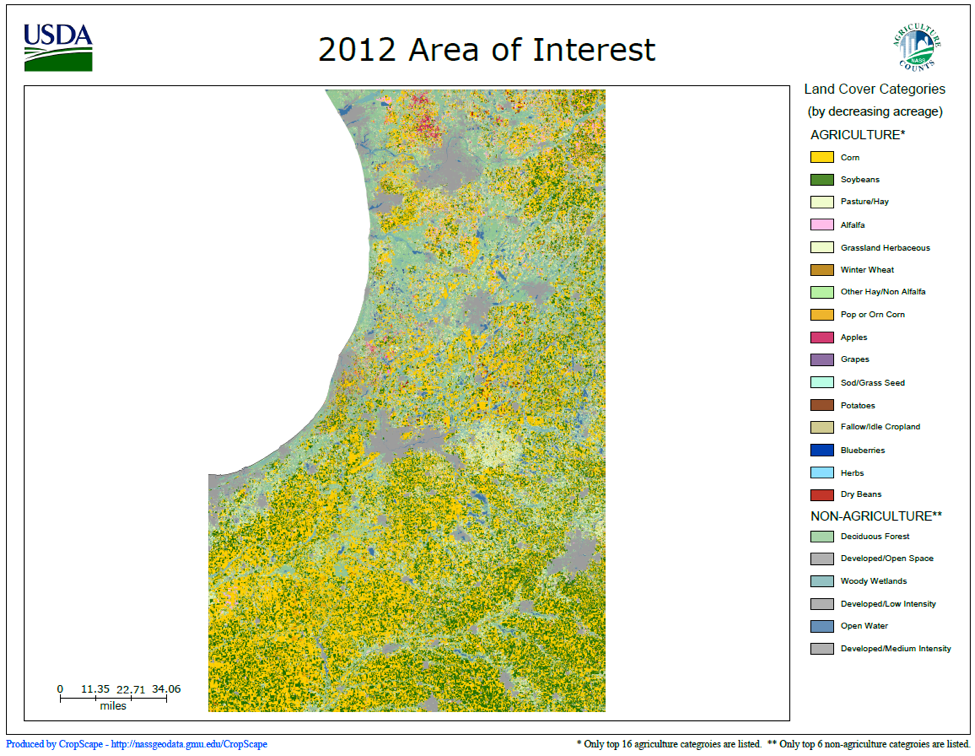
Figure 12. USDA land cover categories for the region in which the Michiana hobby beekeepers keep bees—corn and soy acreage is color coded yellow and green, respectively. The selected area is the top half of Indiana and bottom of Michigan, with Lake Michigan at the left. Clearly, these apiaries were exposed to seed-treated corn and soy! I created the map at [[i]].
[i] http://nassgeodata.gmu.edu/CropScape/
The above figures suggest that colony winter survival for stationary hobby beekeepers in the above corn/soy region is higher than the national average, despite the fact that about half of them don’t even treat for mites! They also suggest that the neonics or other pesticides used in corn/soy in that region do not cause excessive winter loss. Finally, the data indicate that a main factor for winter loss rates is the winter temperature.
Canola
I’ve heard some beekeepers saying that their bees crashed after working canola, suspecting that the seed treatments were the problem. So as a reality check I called a Dakota beekeeper who has been running bees to canola for over a decade—some 10,000 hives last season. He tells me that colony strength after canola varies from year to year, but that he sees no problem with the seed treatments. He did point out that beekeepers should be aware that colonies can plug the broodnest on intense canola flows.
The biology: The plugging out of the broodnest during an intense bloom means that three weeks afterward, there will be few emerging workers to take the place of the worn-out foragers, and the colony population will temporarily plummet. Even worse, the remaining mites are then concentrated onto fewer bees—which can initiate virus epidemics. These colonies must then attempt to rebuild from scratch, starting in August, meaning that the weakened, mite-infested colonies faced three long months of drought last summer for that rebuilding process.
Every field study that I’ve seen for canola also supports the conclusion that the seed-treatments are safe for bees. I joined other beekeepers and regulators in observing a large-scale study of seed-treated canola in Canada [41]. Canola (or rapeseed) is likely the best test crop, since bees eagerly (and virtually exclusively) forage upon it for both pollen and nectar, meaning that every bit of their food supply contains contain easily verifiable residues of the insecticides. The preliminary results indicate that the clothianidin seed treatment did not harm the colonies [42].
Another recent independent long-term field study in Poland [43] came to the same conclusion. In it, the researchers followed 50 colonies for more than two years under field conditions as they foraged on five different large fields of oilseed rape treated with various combinations of five different neonicotinoids applied by seed treatment and spraying. Pollen and nectar samples were taken, and demonstrated that the bees were clearly exposed to normal residues of the insecticides (there was also additional exposure to other common agricultural pesticides). The colonies were monitored for health, brood, strength, nosema, viruses, and winter survival, and compared to two control apiaries set in an area free of the crop. The results?
During the time from the placing of the colonies on the rape fields until wintering, the colonies developed properly in all groups… All colonies overwintered properly… In both years, during the period of being placed in the oilseed rape fields as well as after being moved to the stationary apiary, none of the groups showed disturbances in development or functioning.
Following a paper that suggested that the seed treatments would impair bumblebee colonies’ ability to rear queens, DEFRA performed a common-sense field study last year [44]. Their findings:
…the study has shown that bumble bee colonies remained viable and productive in the presence of the neonicotinoid pesticides under these field conditions…The study underlines the importance of taking care in extrapolating laboratory toxicology studies to the field, as well as the great need of further studies under natural conditions.
Sunflowers
Some beekeepers report that their colonies later crashed after they chased sunflowers last summer for honey. One must keep in mind that sunflowers are not a natural food for honey bees, and provide only poor-quality, nutritionally-inadequate pollen [45]. But the main problem with putting bees on sunflowers may be related to the fact that sunflowers are a native plant—meaning that there are a number of native insects that evolved to feed upon it:
Maximum seed yields often require the use of insecticides to protect the crop from insect competitors. Unfortunately, many of the major insect pests of sunflower attack the crop when it is flowering. Thus, insecticides used to control the pest also harm pollinating bees [46].
If sunflowers are the only forage available, colonies may eventually go downhill, due to the one-two punch of poor pollen nutrition coupled with insecticide exposure. And which pesticides would those be? One scary list– Asana XL, Baythroid, endosulfan, Furadan , Lorsban , methyl or ethyl parathion, , Proaxis, Scout X-TRA, Sevin, Warrior, Mustang Max, Declare, Cobalt, Yuma, Delta Gold, and Grizzly Z [47]!
Note that none of the above are neonics, other than seed treatments for wireworms. Surprisingly, field evidence indicates that the seed treatments only “stun” the wireworms for a while [48], which certainly raises the question as to how harmful they might be to bees months later when the plants flower! I will return to sunflowers below.
Be Careful What You Ask For!
Allow me to assure you that I am no pitchman for neonics or any other insecticide—the typical farmer practices far too little integrated pest management, and applies far too many pesticides! All insecticides (and several fungicides and adjuvants) cause problems to pollinators—the neonics are no exception. Any systemic insecticide has the potential to harm bees when applied as foliar applications, by chemigation, or to flowering trees, but it there is no compelling evidence that the neonics are any worse than the alternatives in most applications. On the contrary, there is quite a bit of evidence that they may often be “safer” (“reduced risk”).
If the neonic seed treatments were banned, it’s not as though all agriculture is suddenly going to go pesticide free—only about 1% of U.S. cropland is registered as “organic”! We must consider the likely alternatives. The products that farmers would then use to control insects would need to be sprayed all over the cropland—we’d then be back to the problem that the bulk of sprayed insecticides go into the environment without ever hitting the intended pest!
I hear from knowledgeable beekeepers that worse than in previous years, some of the new formulations of the spray-applied insecticides [49, 50, 51] can really knock the snot out of bees! One large beekeeper found his hives already dead before moving them away from the fields. Again, this was not a neonicotinoid issue.
Practical application: no one is saying that the neonics are “harmless.” The question is whether they are better or worse than the alternatives.
The Effect of Drought
Let’s discuss some of the problems (or suspected problems) with the neonics last season. The record warm and dry spring appeared to exacerbate corn planting dust issues (corn seeds are the worst offender due to their non spherical shape). Beekeepers in some areas of the Corn Belt, the East Coast, and in Ontario suffered from confirmed (in at least some of the cases) planting dust kills (although many went on to make good honey crops after their colonies recovered). The final analysis from Ontario is not yet completed, but dry soil conditions and an early clover bloom likely contributed to the problem. Regulators and the seed companies are working on solutions to the problem [52]. Still, IMHO it is unacceptable to ask beekeepers to bear the burden of bee kills without compensation, and no one could blame the affected beekeepers for being pissed!
Drought-stressed plants
There are a number of advantages to the neonic seed treatments. Besides their safety to the farmer and to most wildlife, there is virtually no way for the farmer to misapply them! The timing of application is only at planting time (when bees normally have little interest in the bare fields), and the dose is determined by the seed-treating company. This means that the applicator can’t be tempted to apply at the wrong time, or to over apply too strong a dose (however, their excessive near universal use can be expected to accelerate the development of resistant pests).
That said, beekeeper Bret Adee brought an interesting question to my attention: the dose of seed-applied systemic insecticides (whether neonic or other) is based upon the dilution factor as the plant grows, so that the residues in nectar and pollen will be reduced to below the “no observed adverse effects level.” But what happens during drought, when the water-stressed plants only grow knee high before desperately flowering? There would be far less plant biomass in which to dilute the insecticide (assuming that drought-stressed plants absorb the same amount from the seed treatment).
Certain plants (including sunflowers and canola) are known to “hyperaccumulate” toxic metals [53], perhaps more so during drought. Could this also be the case with systemic insecticides? Something that’s been stuck in the back of my mind is that Bonmantin [54] found that the concentration of imidacloprid first drops in sunflower plant tissue as it grows, and then reconcentrates in the flower heads.
It occurs to me that the translocation of systemic insecticides is generally studied in plants grown under “normal” conditions. I’d very much like to see data for residues in pollen and nectar from seed-treated plants grown under drought. Had we thought of this earlier, we could have collected pollen and nectar samples from drought-stressed plants last summer. I’m currently trying to track down any data or samples from such plants—if any reader has any such sample analyses, please let me know!
Practical application: the above hypothesis is speculative, but we need actual data from drought-stressed plants to see whether such an effect occurs. If so, it would need to be taken into consideration for the registration of seed treatment products!
Once planting was completed and the drought took its toll, the reports that I’ve heard are that soybean honey saved a lot of bee operations this season, right in the middle of treated corn/soy farmland. In this case, seed treatment with neonicotinoids may have been a blessing to beekeepers:
The benefits of [seed treatment] not only include the early-season disease control but also suppression of soybean aphids for quite a ways into the growing season. With it, we typically make only one foliar insecticide application for aphid control, usually in August, instead of two applications when [treatment] isn’t used. In 2012, with the extremely dry conditions in mid-season, there wasn’t as much of an aphid problem, and we treated just 300 acres of soybeans…Last year we sprayed closer to 30,000 acres for aphids [55].
On the other hand, some beekeepers on alfalfa or cotton got hit hard by other classes of insecticides. A hit from a pesticide application can lead to poor subsequent colony performance, queen failure, dwindling, or winter collapse. ABJ published an excellent series of articles on pesticides by Drs. Barbara and Eric Erickson in 1983; Editor Joe Graham has graciously granted me permission to post copies of those articles to my website [56]—I strongly suggest any beekeepers interested in pesticide issues read them! In the second article, the authors discuss both the problems with systemic insecticides and of sublethal effects—note that these articles were written long before the introduction of the neonics!
An anti-pesticide group, along with a handful of beekeepers, recently filed suit against the EPA [57], calling for an immediate ban on the two most common neonicotinoid seed treatments, despite the easily-verifiable fact that hundreds of thousands of colonies thrive in the midst of seed-treated corn, soy, and canola! To me, this suit smacks of being some sort of well-orchestrated publicity stunt, and does not serve the interests of either beekeepers or environmentalism. Worse, it now gives the powerful farm lobby cause to label beekeepers as “radical” enemies.
We don’t want this battle: do we really want to take on the farm lobby by backing them into a corner? The French beekeepers took a similar case against fipronil all the way to their supreme court and lost [58, 59]–worth reading]. Agriculture is already positioning itself for a fight [60, 61, 62]. Think about it—the EPA lives in fear of a conservative congress slashing their funding. Does anyone really think that they are going to go against the agricultural lobby without unimpeachable evidence? We should also think twice before calling for a ban on the seed treatments—the alternatives are not pretty!
It disturbs me to hear industry executives and lawyers stretching the truth or misrepresenting data. It disturbs me even more to hear my fellow environmentalists and beekeepers doing so! If we wish to maintain credibility, we should hold ourselves to a higher standard. The question we must ask ourselves the way in which we wish to have pesticide regulation decisions made:
- 1. By the EPA (the Environmental Protection Agency), whose risk assessors carefully study and weigh all available research and evidence in order to make objective and rational decisions, or
- 2. To have it decided instead by impassioned, fearful, and often misinformed advocacy groups who hire lawyers and pressure politicians who know little about the subject?
We depend upon the EPA to strike a balance between the availability of cheap food and profitability for those who provide it, versus the risks to human and environmental health and safety. It is good to have activists on both sides of the issues (industry and the anti-pesticide groups) to keep the EPA informed. But I don’t feel that either of those groups should be telling the EPA which pesticides to register or to ban! Let the regulators do their job!
Rather than wasting EPA’s funding to fight frivolous lawsuits, there are more productive actions that we can take:
- Help the EPA to do its job by filing “adverse effects incident reports” if you observe a problem due to pesticides [63]. EPA is begging beekeepers to do this! Unless they have documented reports of pesticide problems, their hands are tied as to restricting the uses of those pesticides!
- Support the National Pollinator Defense Fund [64]. Our industry is currently represented by a reasoned and knowledgeable group of (mostly) beekeepers. (Challenge to the pesticide companies: why don’t you stand behind the safety of your products and donate? The NPDF is about ensuring that your pesticides are properly applied, so there would be no conflict of interest).
- If your local state lead agency is not actively investigating bee kills or enforcing pesticide regulations, then use the local media to embarrass them into action!
- Keep pressure on the EPA to resolve corn planting dust problems. Here’s a wild idea: I’m not sure of the exact figures, but let’s say that 90% of the 95 million acres of corn is grown from neonic-treated seed. If the states were to levy a surcharge of 50 cents per acre (neonic seed treatment adds about $12 per acre to seed costs), they could collect over $42 million each year to fund a pool from which to indemnify the occasional beekeeper who suffers a confirmed kill from planting dust!
- Tell Congress that we’d like to see wording added to the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) to specifically protect pollinators. Currently, such protection is nebulous (although the EPA is acutely aware of pollinator issues): “The Administrator shall register a pesticide if… when used in accordance with widespread and commonly recognized practice it will not generally cause unreasonable adverse effects on the environment.” Unless there is specific wording to protect pollinators, bee kills may not be considered to be “unreasonable”!
- We need far more independent field studies to determine which pesticides and application practices are actually causing harm to pollinators. For pesticides in question, keep pressure on the EPA to require additional field trials to demonstrate whether they are indeed safe for pollinators under field conditions. I’d like to see the establishment of monitoring apiaries (and patches of untilled land) in representative agricultural areas nationwide, with the hives in each apiary to be carefully managed by independent parties. Such apiaries and sites could then be closely monitored each summer to see whether honey bees and other pollinators are able to survive local pesticide practices.
- Give farmers workable options! Disseminate and promote bee-friendly agricultural practices that don’t hurt the farmers’ bottom line. For example, by adopting IPM practices, Arizona cotton growers reduced insecticide spraying from 12.5 times a season to only 1.3 times (cutting insecticide use twentyfold), while using more environmentally-friendly insecticides [65]! Another recent study in Iowa found that adding additional clover or alfalfa rotations in corn/soy farmland was equally profitable, improved the soil, used less energy, used far less pesticides, and decreased water pollution [66].
- Business and agriculture respond to consumer demand. Consumer demand stopped most dairymen from injecting their cows with the hormone BST. Consumers could do the same by demanding pasture-fed beef and dairy (which would create more pollinator forage)! I’d also like to see the expansion of consumer choices (other than organic certification) that reward farmers who manage their lands to the benefit of wildlife and pollinators. For ideas, see [67. 68. 69].
Bottom Line
In conclusion, it appears that a perfect storm of a preceding exceptionally warm winter, followed by serious drought across the country, the lack of good mite control, a high prevalence of pathogens, and an unexpected California chill in the orchards prior to bloom, resulted in an unusual degree of colony losses. In other words, rather than one specific cause, there were simply not enough of the good things, and too many of the bad things.
I don’t see evidence that pesticides were the major factor in the shortage of bees in almonds this winter, although, as usual, a number of individual beekeepers on certain crops certainly took serious hits.
And how about the fear that there won’t be enough bees for almond pollination next year? Beekeepers have already told almond growers to expect higher pollination prices next year (especially since California is again going into serious drought, and beekeepers will be forced to invest extra money in feeding their hives). Most every beekeeper I know is madly making increase right now in anticipation of higher pollination prices next season. The fact of the matter is that should conditions allow beekeepers to successfully rebuild their numbers (following the typical swings of our boom/bust cycle), there could possibly even be a glut of bees for almonds next winter!
Feedback and Corrections
- A few countries placed temporary suspensions on certain seed treatments until planting dust issues were resolved [27]—only Germany has one suspension still in place. – Actually there are some more: France (Thiamethoxam in oilseed rape, Imidacloprid in corn and sunflower), Italy (all Neonic seed treatment in corn), and Slovenia (Imidacloprid, Thiamethoxam, and Clothianidin seed treatment in all crops)
- French beekeepers also started seeing problems with the introduction of the neonics; I’ve spoken with beekeepers in France whose apiaries are in pesticide‐free areas. They tell me that they experience the same sorts of colony mortality problems as do those in areas exposed to neonics – This is, by the way, likewise confirmed by monitoring results from the French authorities.
- In the case of foliar, drench, or chemigation applications prior to bloom, there are greater possibilities for bees to be exposed to toxic levels. – This is not the case for foliar application: as Neonics are xylem-systemic, but hardly mobile in the phloem, they can only be distributed in a plant after root uptake, but not be translocated for instance from a leaf to a later developed flower.
Then, on the topic of systemic residues in plants under drought stress: first, I am quite sure that the decrease of concentration in seed-treated plants over time is not only due to dilution, but also to degradation of the compounds – a factor that is not specifically dependent on water availability for the plants (e.g. photodegradation!); second, even if there would be less dilution in plants under drought stress: the concentrations in nectar and pollen of treated crops are normally so low (when we consider average rather than peak concentrations, and when we consider scenarios where colonies have chronically access exclusively to contaminated nectar/pollen over months unlikely in practice), that even an increased concentration due to drought stress-affected plants should not make a significant difference: if we for instance assume an average concentration of let’s say 3-4, or even 5 ppb Clothianidin in corn pollen, and likewise assume a dilution reduced to 50% (which is probably exaggerated), then we would still not end up with excessive residues. And finally, we have residue figures from crops grown in different countries, different climatic conditions, and different agronomic practices; though we have not specifically addressed the drought stress scenario, we have seen that residue figures are quite consistent over all scenarios, and there does not appear to be strong evidence that different environmental conditions would substantially (i.e. by orders of magnitude) and systematically alter residue concentrations.
Dr. Christian Maus
Global Pollinator Safety Manager
Bayer CropScience
/
A ppt on the impact of CRP lands on wildlife in North Dakota
http://www.redriverbasincommission.org/Conference/Proceedings/26th_Proceedings/Kading_RRBC_09.pdf
/
Feedback from a Midwestern apiary inspector:
Just a quick update: This beekeeper who recently told his local newspaper that pesticides were killing his bees, he was making excuses. We examined two of his yards with him yesterday. One yard was showing EFB throughout the whole yard. I think his “mid-summer losses” last year (half of that yard’s hives) were EFB kicking in with the mid-summer dearth. In his other yard, most of his dead outs were obvious starve outs. He harvested all of their stores with the first frost, and then didn’t feed them. So, in my opinion, and from my observations, pesticides are usually being used responsibly, and aren’t killing honey bees. I also think, with the aggressive way bees were on soy fields last summer, the systemic pesticides are not harmful to honeybees. I’m not seeing honey bee problems other than EFB getting the upper hand, due to our cold, late spring. My two cents. Thanks.
References
[1] http://www.frequency.com/video/dan-rather-reports-buzzkill/87705620/-/YouTube
[2] http://www.ars.usda.gov/is/pr/2012/120531.htm and California DPR.
[3] http://www.ncdc.noaa.gov/temp-and-precip/maps.php
[4] Wright, CK & MC Wimberly (2013) Recent land use change in the Western Corn Belt threatens grasslands and wetlands. https://www.motherjones.com/files/pnas201215404_nwow5w1.pdf
[5] http://www.aphis.usda.gov/plant_health/plant_pest_info/honey_bees/downloads/2011_National_Survey_Report.pdf
[6] https://scientificbeekeeping.com/sick-bees-part-2-a-model-of-colony-collapse/
[7] vanEngelsdorp, D, et al (2013) Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Preventive Veterinary Medicine 108(2-3): 225-233. http://www.sciencedirect.com/science/article/pii/S0167587712002656
[8] https://scientificbeekeeping.com/sick-bees-part-18a-colony-collapse-revisited/
[9] http://almondinsights.com/692, http://agfax.com/almonds/2013/reports/03042013-almonds-web.htm
[10] https://scientificbeekeeping.com/sick-bees-part-18a-colony-collaspse-revisited/
[11] http://www.aphis.usda.gov/plant_health/plant_pest_info/honey_bees/downloads/2010-2011-Limited_Survey_Report.pdf
[12] http://www.youtube.com/watch?v=y6B5qm2ut18, http://www.youtube.com/watch?v=PYbLbhZXizY
[13] (Broken Link!) http://www.nccrops.com/2012/07/27/insecticide-recommendations-for-corn-earworm-in-soybeans/
[14] http://www.agriview.com/news/crop/corn-soil-insecticide-use-up-dramatically-to-combat-widespread-rootworm/article_5d09decc-5b40-11e2-b485-001a4bcf887a.html
[15] http://www.amvac-chemical.com/products/documents/Counter20G%20Tech-Sell%20Sheet%20-%202013.pdf
[16] http://pmep.cce.cornell.edu/profiles/insect-mite/propetamphos-zetacyperm/terbufos/insect-prof-terbufos.html
[17] http://www.lewishybrids.com/PDF/3-5-2013Agronomic+ALERT+-+Interaction+between+herbicides+insecticides+corn.pdf
[18] http://pest.ca.uky.edu/EXT/Recs/ENT16-Field%20corn.pdf
[19] (Broken Link!) http://www.soybeans.umn.edu/crop/insects/spider_mites.htm
[20] http://www.nytimes.com/2013/04/07/opinion/sunday/calamity-for-our-most-beneficent-insect.html?nl=todaysheadlines&emc=edit_th_20130407&_r=0
[21] http://www.nytimes.com/2013/04/07/opinion/sunday/calamity-for-our-most-beneficent-insect.html?_r=0
[22] http://www.panna.org/press-release/beekeepers-and-public-interest-groups-sue-epa-over-bee-toxic-pesticides
[23] http://www.cdpr.ca.gov/docs/pur/pur10rep/top_100_ais_lbs10.pdf
[24] http://www.extension.org/pages/60318/pesticides-and-their-involvement-in-colony-collapse-disorder
[25] Mussen, EC (2006) Chaotic almond pollination. http://entomology.ucdavis.edu/faculty/mussen/JanFeb2006.pdf
[26] https://scientificbeekeeping.com/sick-bees-part-18e-colony-collapse-revisited-genetically-modified-plants/
[27] http://www.epa.gov/pesticides/about/intheworks/ccd-european-ban.html
[28] http://www.thecre.com/oira_pd/wp-content/uploads/2013/04/DQA-Alert-EU-Commission-Ban-on-Neonicotinoids-4-10.pdf
[29] https://scientificbeekeeping.com/home/news-and-blogs/
[30] Henderson, CB, JJ Bromenshenk, DL Fischer (2013) Clothianidin exposure levels from bee-collected pollen and nectar in seed-treated corn and canola plantings. Proceedings of the American Bee Research Conference.
[31] http://www.aphis.usda.gov/plant_health/plant_pest_info/honey_bees/downloads/2011_National_Survey_Report.pdf
[32] http://www.ontariograinfarmer.ca/MAGAZINE.aspx?aid=534
[33] http://www.pbs.org/wgbh/pages/frontline/shows/settlement/timelines/april94.html
[34] https://scientificbeekeeping.com/2012-almond-pollination-update/
[35] https://scientificbeekeeping.com/neonicotinoids-trying-to-make-sense-of-the-science/, https://scientificbeekeeping.com/neonicotinoids-trying-to-make-sense-of-the-science-part-2/, https://scientificbeekeeping.com/testing-of-bee-feed-syrups-for-neonicotinoid-residues/
[36] http://www.eea.europa.eu/publications/late-lessons-2
[37] http://www.sciencefriday.com/playlist/#play/segment/9088
[38] https://scientificbeekeeping.com/the-extinction-of-the-honey-bee/
[39] http://www.ncdc.noaa.gov/temp-and-precip/maps
[40] http://nassgeodata.gmu.edu/CropScape/
[41] https://scientificbeekeeping.com/a-new-large-scale-trial-of-clothianidin/
[42] http://www.producer.com/daily/ontario-field-study-finds-no-link-between-seed-treatments-bee-deaths/
[43] Pohorecka, K, et al (2013) Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. Journal of Apicultural Science 56(2): 115-134. http://www.degruyter.com/view/j/jas.2012.56.issue-2/v10289-012-0029-3/v10289-012-0029-3.xml?format=INT
[44] http://www.fera.defra.gov.uk/scienceResearch/scienceCapabilities/chemicalsEnvironment/documents/reportPS2371Mar13.pdf
[45] http://repository.up.ac.za/bitstream/handle/2263/20334/Nicolson_Chemical(2012).pdf?sequence=1
[46] (Broken Link!) http://www.ag.ndsu.nodak.edu/aginfo/entomology/entupdates/Sunflower/a1331sunflowerhandbook.pdf
[47] http://www.sunflowernsa.com/growers/approved-chemicals/insecticides-test/
[48] http://www.mydigitalpublication.com/publication/?i=151958&p=41
[49] http://www.sunflowernsa.com/growers/approved-chemicals/insecticides-test/
[50] http://www.farmassist.com/agriedge/images/Resource_PDFs/Soybean/Warrior_Zeon.pdf
[51] http://www2.dupont.com/Production_Agriculture/en_US/assets/downloads/pdfs/K-09315.pdf
[52] http://www.ontariograinfarmer.ca/MAGAZINE.aspx?aid=534
[53] http://en.wikipedia.org/wiki/List_of_hyperaccumulators
[54] Bonmatin, JM, et al (2005) Behaviour of Imidacloprid in Fields. Toxicity for Honey Bees. In Environmental chemistry: green chemistry and pollutants in ecosystems pp. 483-49. http://www.buzzaboutbees.net/support-files/bonmatin2005behaviour-of-imidacloprid-in-fields.pdf
[55] http://cornandsoybeandigest.com/seed/do-soy-seed-treatments-pay?page=2
[56] https://scientificbeekeeping.com/historical-pesticide-overview/
[57] http://www.centerforfoodsafety.org/press-releases/1911/cfs-beekeepers-and-public-interest-groups-sue-epa-over-bee-toxic-pesticides
[58] http://www.theworldlawgroup.com/files/file/docs/Soulier_health_environment_June_2012.pdf
[59] http://www.soulier-avocats.com/upload/documents/Soulier_health_environment_september_2010_F.pdf
[60] http://westernfarmpress.com/government/pesticide-battle-over-honey-bee-health-under-way?page=1
[61] http://westernfarmpress.com/management/total-ag-pesticide-elimination-sought-radicals
[62] http://www.neonicreport.com/home/project-compass/
[63] https://scientificbeekeeping.com/pesticide-incident-reporting/
[64] http://pollinatordefense.org/site/
[65] http://cals.arizona.edu/apmc/docs/IPM_Delivers.pdf
[66] Davis, AS, et al (2012) Increasing cropping system diversity balances productivity, profitability and environmental health. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0047149
[67] http://www.pcl.org/pcl_files/5_Wildlife_Habitat_Farmland.pdf
[68] http://pfspbees.org/
[69] http://www.nwf.org/CertifiedWildlifeHabitat/UserAccount/SignIn























