Refining the Mite Wash: Part 4 – Comparing the Release Agents
Refining the Mite Wash : Part 4
Comparing the Release Agents
Randy Oliver
ScientificBeekeeping.com
First Published in ABJ in October 2020
As I tested different release agents for varroa monitoring, I was often surprised by the results, which then raised new questions about why some worked better than others. So I ran a number of experiments in order to tease out possible answers.
Varroa Management is about Reducing Virus Transmission
Varroa mites build up in colonies at a predictable rate (easy to visualize with my recently-updated interactive mite model [[1]]). Similar to dealing with the coronavirus, an in-hive Deformed Wing Virus epidemic can be prevented by suppressing the transmission rate of the virus within a hive. That can be accomplished by keeping the varroa infestation rate (mites per hundred bees) low at all times of the year.
Practical application: The best way to deal with varroa is to be proactive rather than reactive — keep in mind that the main problem with varroa is that it is the vector for Deformed Wing Virus. Your colonies will be healthier and more productive if you never allow infestation rates to climb above around the 2-3% level (6-10 mites in a mite wash or sugar shake). As with the coronavirus, it’s easier to nip the epidemic in the bud, rather than trying to wrestle varroa/DWV back under control after they’ve established a raging infestation.
What with the coronavirus epidemic, we’re hearing about testing for the prevalence of infected individuals. This is exactly the same reason for performing mite washes — to determine the prevalence of mites in a sample of a half cup of bees. What we want is an “accurate” test — one that doesn’t miss any mites (its sensitivity), nor overcount mites due to misidentification.
There are two parts to the mite wash test:
- The mechanics — efficiently separating the mites from the bees with a release agent, agitation, and precipitation through a screen,
- Accurate counting (involving an error-prone human) — which involves putting on your reading glasses so that you don’t miss any mites (false negatives) or count hive trash as a mite (false positives).
Practical application: In my experience, beekeepers commonly fail on both counts — using poor method or technique for the wash, and then not correctly recognizing mites. When doing field demonstrations I often perform a mite wash, silently count the mites (let’s say 5), write it on my palm, and then pass the cup around for others to count out loud. I nod as I hear counts ranging from 1 to 15 mites from the same cup! Then I hand the cup to a twenty-something, who invariably calls out the same count as on my palm.
So test yourself. Put on your reading glasses, and run a few mite washes, saving the mites (Figure 1) until you have 10 in total. Then shake those 10 mites back on top of the last sample of bees, rewash them, and see if you get a count of 10 again. Repeat a few times until you are confident in your technique.
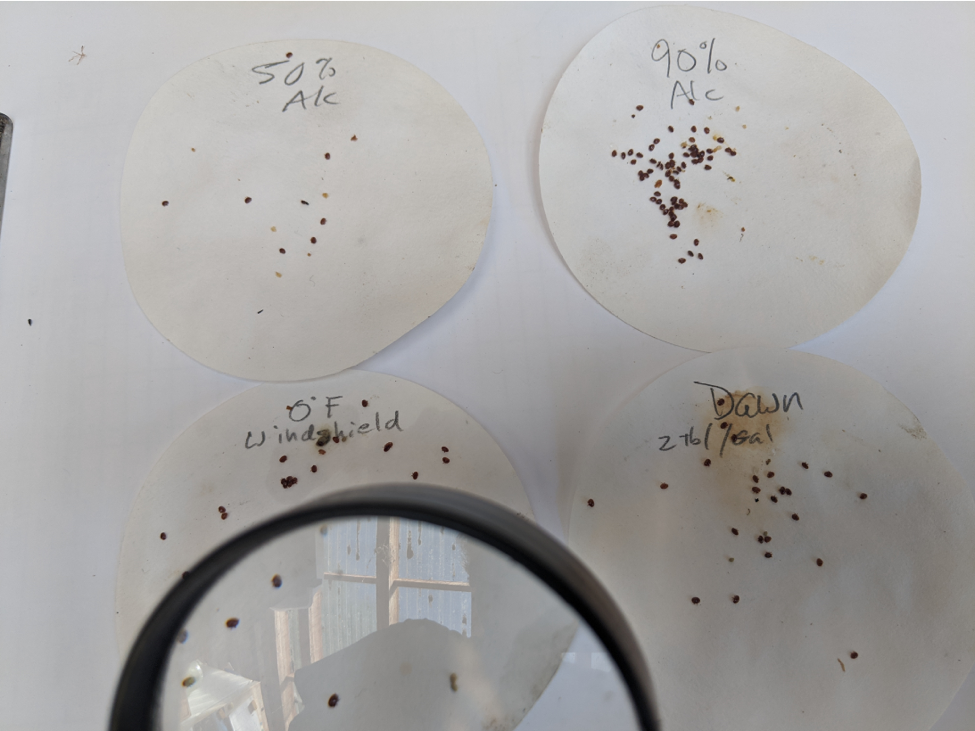
Fig. 1 Recovered varroa mites are brownish-orange, oval, with eight legs, and all roughly equal in size (smaller or larger objects are not mites). A magnifying mirror makes them easy to see from below. Note the mite on edge, the fourth in from the right.
Once you’ve gotten your mechanics and counting method down, now it’s up to using a good release agent (powdered sugar or a liquid). Since we do a lot of mite washes in our operation (well over 2500 a season), I personally want a release agent that consistently gives me at least 95% mite recovery with only 60 seconds of agitation (mechanical in my case). Since we reject potential breeder queens if their colony’s mite count is over 1 at the first assessment, it’s imperative that we recover every single mite.
So when alcohol became scarce due to COVID, I decided to test other release agents.
Questions Raised
After some preliminary testing, I had a number of questions about why certain liquid release agents worked better than others:
- How quickly does the liquid immobilize or kill the mites or the bees?
- Was the immobilization due to toxicity of the liquid?
- Do the mites release due to irritation by the liquid, or is it something else?
- Or is it due to the wetting (surfactant) action?
- Does it make a difference that bees float in some liquids, but sink quickly in others?
- Is there a value to delaying agitation once the bees are submerged?
In order to answer the above questions, I performed experiments involving over 400 washes in all (that’s over 30 pounds of mite-infested bees).
Practical application: In order to calculate sensitivity (or to test the efficacy of treatments) a common mistake is to use bees with low infestation rates. The problem in doing so is that the 100% difference between a mite count of 1 or 2 has far too much inherent error. So I perform my testing with bees from colonies with sky-high counts, from which I can determine meaningful differences in results. To that end, I ask my sons to intentionally allow some second-year colonies to go untreated, for me to use for testing and experiments. That means that we must sacrifice many colonies each year, and appreciate the donations from beekeepers to help us to cover that cost.
Testing of Release Liquids
In order to answer the above questions, I chose for preliminary testing liquids that had different alcohol concentrations, degree of toxicity, strong or weak surfactant value, or contained known mite irritants (Figure 2).
Fig. 2 I chose a range of liquids for my preliminary testing, each with different properties. Not shown are additional liquids added after the preliminary testing (-15°F windshield fluid, and two additional detergents).
Surfactants, Detergents, and Foaming Agents
Due to their water-repellent exoskeletons, bees or mites dumped into plain water float on the surface for a while. So we need a release liquid that deals with that. Alcohol is not only a great solvent, but it also reduces the surface tension of water, thus acting as a wetting agent (proportional to its concentration). This wetting action causes bees and mites to quickly sink, and is likely responsible for disrupting the grip of the mites’ empodia upon the bees.
There are other agents that reduce surface tension, collectively called surfactants. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The foaming agents allow bubbles to form at the air-water interface, with Dawn dishwashing liquid being well known to soap bubble aficionados [[2]]. (Note that alcohol does not cause foaming.)
Other detergents are known for their cleansing properties or ability to suspend oil and grease in water by forming micelles (such as low-foaming Cascade automatic dishwasher detergent). This property also disrupts cell membranes (the industrial detergent Triton x-100 is commonly used for this purpose).
In Part 1 of this series of articles I mentioned previous research by De Jong [[3]] and Rinderer [[4]] that indicated that one could successfully use detergent for mite washes. But not all detergents are the same. I had no idea whether it was the foaming action, wetting action, “cleansing/oil emulsification” action, or cell membrane destruction that most affected mite drop, so I eventually tested a variety of detergents, each with different properties (Figure 3).
Fig. 3 In addition to Dawn, I tested Cascade and Triton x-100 (widely used in labs and industry).
Practical application: I performed a number of runs to determine the optimal dilution of Dawn Ultra (I used the clear Lemon Essence). At less than 1 tablespoon/gallon, mite recovery dropped off. Above 2 Tbl/gal, it didn’t improve. So the optimal dilution appears to be in the 1-2 Tbl/gal range. For the others, I used 2 Tbl/gal of Cascade, and the 2% solution of Triton x-100 recommended for general use.
Dawn Ultra contains two surfactants [[5]], sodium laurel sulfate and sodium laureth sulfate (both have a number of alternate chemical names). These surfactants are commonly used as foaming agents and detergents, and are relatively safe to use (they are common ingredients in toothpaste and shampoo) and biodegradable [[6]]. Just don’t dump them directly into waterways, as any surfactant can harm aquatic life.
Irritants
I was curious as to whether adding an irritant would improve the mite drop due to a surfactant. Listerine fit the bill, since it contains not only alcohol and a surfactant (Figure 4), but also four compounds known to be irritating and toxic to varroa (Figure 5).
Fig. 4 Listerine clearly contained a foaming agent.
Fig. 5 Listerine also contains a goodly amount of ethyl alcohol, which allows it to dissolve small amounts of the oils of eucalyptus, mint, wintergreen and thyme, all well-known to be irritating and toxic to varroa mites. I wondered whether they would cause the mites to release and/or die.
Windshield Washer Liquid
Although not all the ingredients are listed on the bottle, low-temp windshield washer fluid is said to be typically made of distilled water, methanol as a solvent and antifreeze, a surfactant or detergent for cleaning, and coloring. Approximately 20-25% methanol is typically added to keep the fluid in liquid form at 0°F (-18°C). I was surprised by the minimal foaming action of the two types that I tested (Figure 6).
Fig. 6 In California, the lowest temperature windshield fluid allowed for sale is -15°, which it took me a while to find. In this photo, you can see the stand for counting mites with a magnifying mirror in the background (the wash cup fits into the ring above the mirror). The windshield fluids that I tested exhibited little wetting action on the bees.
Instructions for fabricating the wash cups shown above can be found at [[7]].
Immobilization Action and Toxicity to the Mites
Is it important to kill the mites to get them to release? In order to determine the toxicity of the liquids to varroa, I agitated bee samples for 60 seconds in each liquid, then poured the dropped mites into a sieve and immediately rinsed them with water (Figure 7).
Fig. 7 Rinsing the mites with water after a minute of agitation in the test liquid.
I then shook the mites onto filter paper and allowed them to air dry, inspecting them at regular intervals for signs of movement (Figure 8).
Fig. 8 There were large differences in the degree of immobilization of the mites, as well as in how long it took any survivors to recover and walk away.
The results are summarized below (Figure 9).
Fig. 9 The mites laughed at the windshield fluid and 50% alcohol, died in 91% alcohol, very slowly recovered after immersion in Dawn, but surprisingly, most quickly recovered from Listerine.
Practical application: My testing indicated that killing the mites does not appear to be critical to getting them to release.
Do the Bees and Mites Drown?
It is commonly said that one can use a soap or detergent solution to drown bees. But that didn’t make sense to me, since the bees die in less than a minute in a solution of Dawn, whereas I knew that bees could hold their breath for much longer. So I dumped equal amounts of bees into diluted Dawn or plain water, and held them underwater with a screen, jiggling them to remove air bubbles. After 60 seconds of immersion without access to air, I dumped them out on paper (after quickly rinsing the Dawn-exposed bees with water), and waited to see how many of each group survived (Figure 10).
Fig. 10 I dumped equal amounts of bees, at the same time, into the rings, and placed the paper over the top bars for several minutes to allow any live bees to return to the hive. I didn’t run statistics, but there was a dramatic difference between the two test groups. Bees in Dawn clearly don’t die due to “drowning.”
As explained by Colorado State Extension [[8]]:
How soaps and detergents kill insects is still poorly understood. In most cases, control results from disruption of the cell membranes of the insect.
I suspect that the surfactant action of detergents allows the solution to penetrate the bees’ and mites’ spiracles, and then once inside their trachea, disrupt their cellular membranes, causing rapid death. A search of the literature found a study that came to a similar conclusion [[9]]:
Mites immersed in 0.2% Triton X-100 died rapidly … We postulated that the hypo-osmolarity of the distilled water caused an osmotic influx of water into the mite tissues and this was exacerbated in the presence of Triton X-100 which may have allowed water to enter through the spiracles more rapidly with the reduced water tension.
So at this point, I needed to run more tests.
Mite Release without Agitation
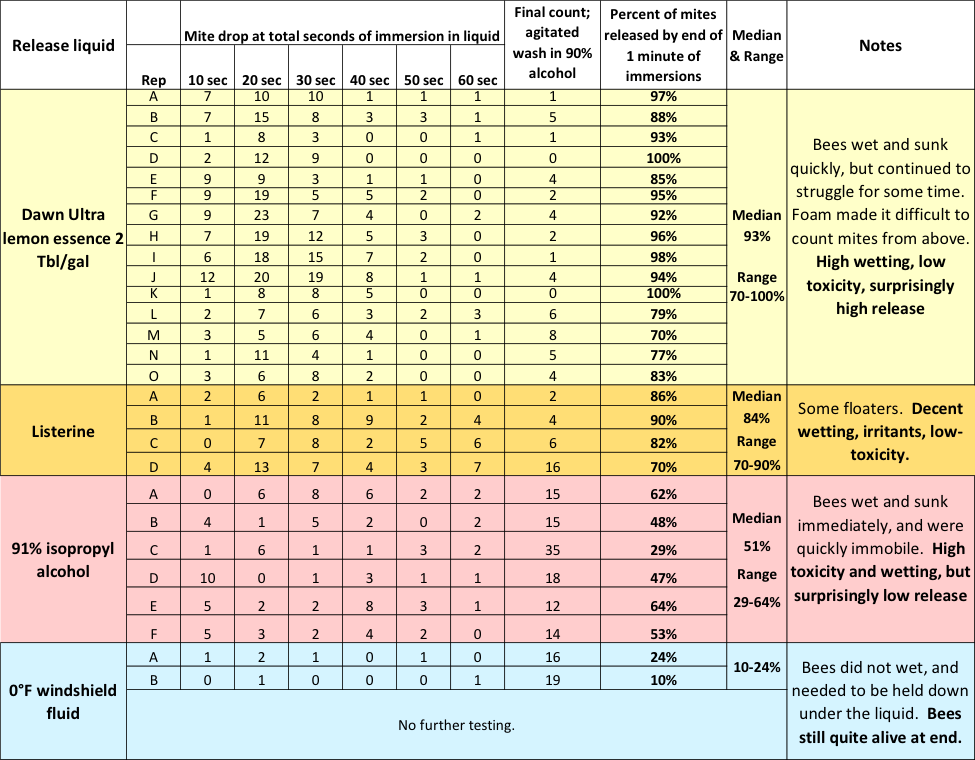
Still curious, I tested Dawn, Listerine, 91% isopropyl alcohol, and windshield fluid for the degree of mite release without agitation. I set up a row of six cups, filled each with whatever fluid I was testing, and then sequentially immersed the same sample of about a quarter cup of bees 10 seconds at a time down the row of cups (Figure 11).
Fig. 11 After the bee sample had gone through the six cups, for a total of 60 seconds of immersion, I agitated them for 300 revolutions in 91% alcohol for final mite recovery. The results are shown below (Table 1).
Table 1 Note the progression of mites dropped during each 10 seconds, without agitation, over the course of a total of 60 seconds of immersion in different liquids. With Dawn, I obtained a median total mite drop, without agitation, of 93%. The detergent considerably outperformed 91% alcohol (median 51% drop), which greatly surprised me.
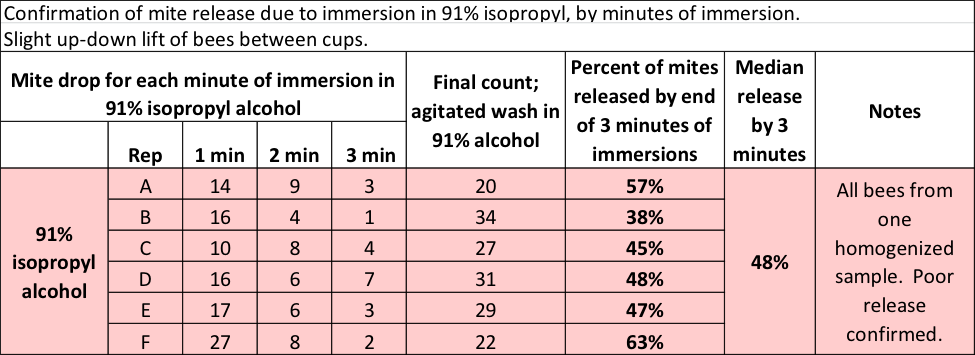
So could Dawn actually outperform 91% alcohol? Incredulous, I ran another test, this time allowing the bee sample to sit in the 91% alcohol for a full minute at a time, repeating for a total of 3 minutes of immersion (Table 2).
Table 2 Even with three minutes of immersion in 91% alcohol, only half the mites hit the bottom.
It was clear that Dawn was outperforming alcohol. But how?
Why the Difference in Results with Alcohol?
I was flummoxed by why 91% alcohol performed so poorly, after getting nearly twice the rate of mite drop in a previous test. And then I realized that in my previous test, the bees were only one layer deep. The difference was apparently due to how thick the layer of bees is that the mites need to precipitate through after they release their grip.
- In 91% alcohol, the bees and mites wet quickly and sink until they hit the screen, where they lie immobile. As the mites continue to release, half of them drop through the screen, but those that drop from the upper layers of bees get stuck in the bee bodies below.
- In Dawn detergent, the bees wet, but take a while to sink. During the first 30 seconds, they move around. That apparently allows the mites to precipitate down through the moving bees. When I do the math for the Dawn test in Table 1, a median of 88% of the mites dropped from the bees without agitation in the first 40 seconds.
Practical application: When using Dawn, most of the mites drop to the bottom of the cup in the first 60 seconds, due to the movement of the bees themselves. After that, only minimal agitation — swirl action, not shaking — is necessary to precipitate the rest of the mites. Take-home: Allow the bees to set in the cup for at least a minute before you begin agitation.
Results of the Tests
OK, now to the real meat of the issue — how do the various liquids compare when you add agitation? I used my mechanical agitators to provide a standardized circular agitation of 300 revolutions over 60 seconds.
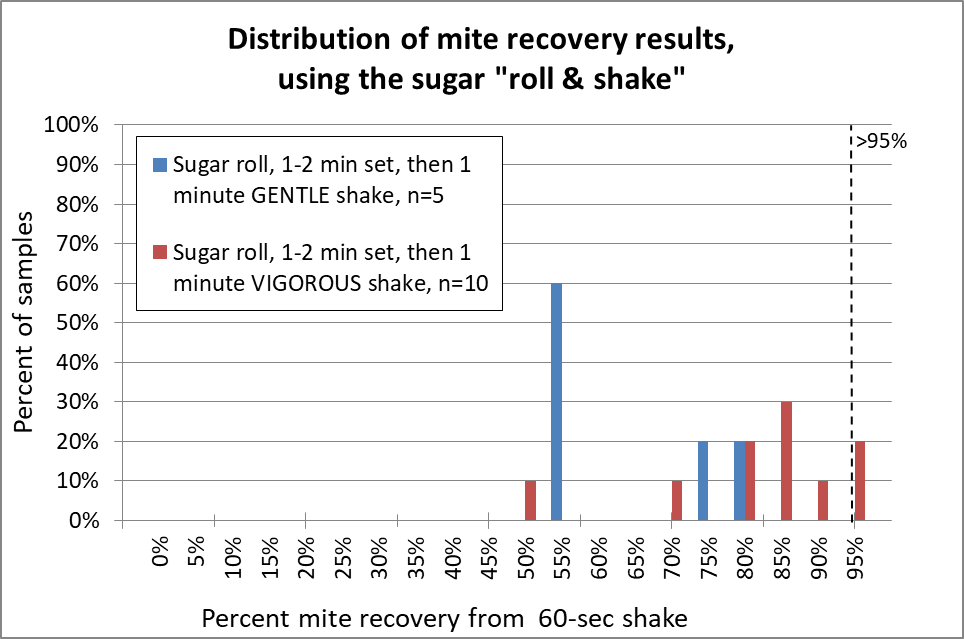
The graphs below summarize over 130 different mite agitations. Rather than giving you a bunch of numbers, box plots, and statistical analysis, I decided to present the data as normalized histograms (so each color of columns adds up to 100% of the reps for each release agent). A histogram is a frequency distribution, allowing anyone to use their own eyes to detect a pattern, and to see the amount of variation. The more sensitive the release agent, the further to the right the columns will be. I’ve included for reference a vertical dotted line to indicate recovery of better than 95% of the mites (Figures 12-15).
Fig. 12 The sugar roll clearly requires vigorous shaking (red) in order to recover a high percentage of the mites; gentle shaking (blue) doesn’t do it. Note that even when I carefully performed sugar roll/shakes with very vigorous up-down shaking, in two of the samples I obtained 70% or less mite recovery, which might seriously underestimate the actual infestation rate. In only two of ten reps did I obtain 95% mite recovery.
My recovery was not nearly as good as that of Vesco [[10]], who claimed 94% mean (instead of median) mite recovery, but from low-mite colonies. My results were more in line with those of Macedo [[11]] for high-mite samples, but much better than that of Flores [[12]]. Anyway, I really gave the sugar shake a good shot, but was not impressed. So how about alcohol?
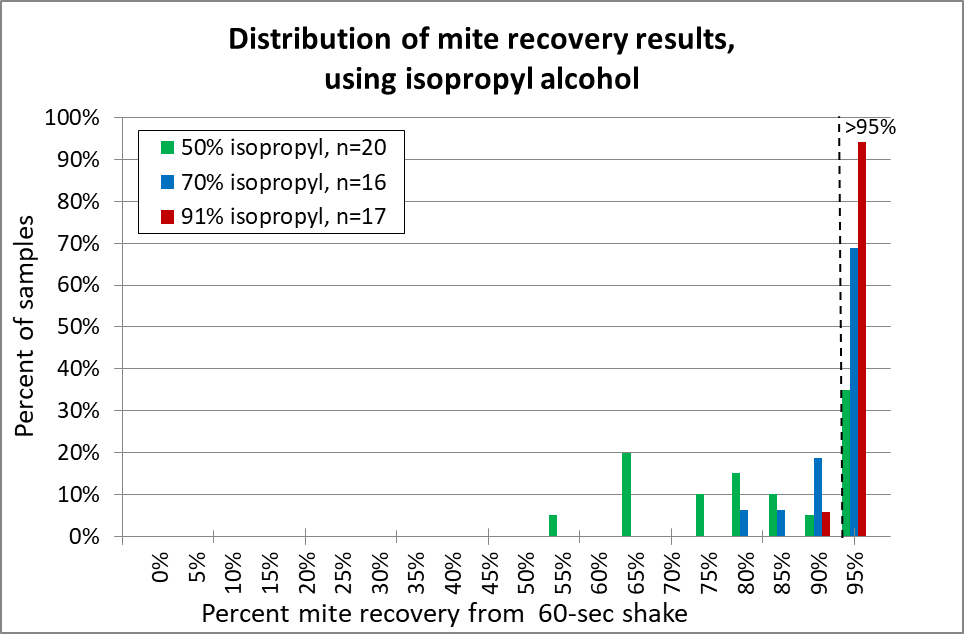
Fig. 13 With isopropyl alcohol, 91% (red) was the clear winner. I suggest that it be the standard (as opposed to 70% alcohol (blue) to which other release agents should be compared. 50% alcohol (green) is unreliable.
Practical application: 91% isopropyl alcohol is a bit pricey, is restricted for “organic” certification, and is flammable. So it’s worthwhile to look for alternatives.
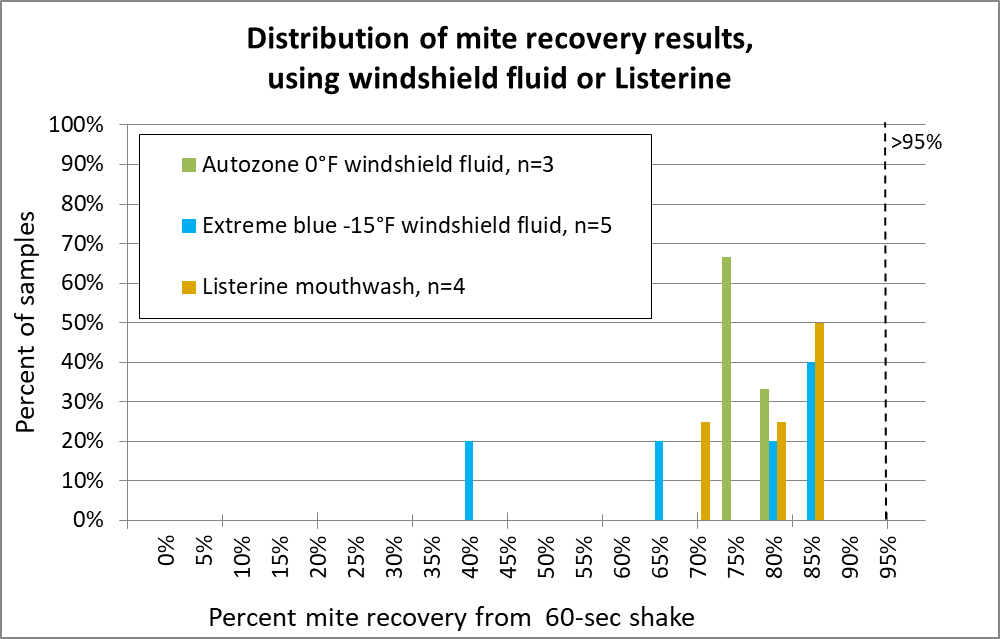
How about two home products — windshield fluid or Listerine?
Fig. 14 I wasn’t impressed enough by windshield fluid to run many samples. And Listerine, despite its four irritating essential oils, also fared poorly. Canadians use a lower-temp windshield fluid, but one researcher told me that it was necessary to add additional alcohol for good performance.
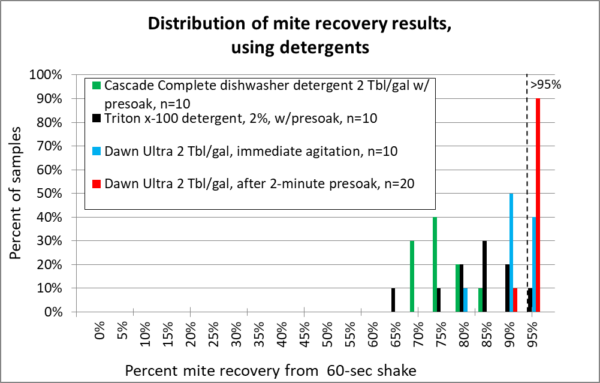
So let’s move on to the detergents that I tested (no need to ask me about others, since I haven’t tried them). I allowed the bees to soak in the detergent first for a minute before agitation, and also compared Dawn with immediate agitation to see how much difference it made to wait.
Note: Thanks to the keen eye of reader ShawnM, the concentrations used for the Dawn runs have been corrected from the original article. They are only 0.8%.
Fig. 15 The detergents. Low suds Cascade (green) really cleaned my equipment, but provided poor mite recovery. Triton x-100 (black) was a bit better, but didn’t make grade. For Dawn, note that I ran two different tests — one with immediate agitation (blue), the other after allowing the bees to first soak for two minutes (red). The presoak made quite a difference!
These results confirm those of De Jong and Rinderer that detergent can work well for mite washes, provided that you use the right detergent, and allow enough time for the mites to release [[13]].
The Winners
Isopropyl alcohol at 70% concentration has long been considered to be the “gold standard” for mite washes. But the 91% concentration is clearly more effective.
To my surprise, one dishwashing liquid, Dawn Ultra, not only performed equally as well as 91% isopropyl alcohol, but requires less agitation, is cheaper, and is non-flammable. We’ve now performed well over a thousand mite washes with Dawn, and are very pleased with it. I’m not stuck on Dawn — I suspect that any foaming detergent containing enough sodium lauryl and laureth sulfate would work well.
Practical application: For best results, allow the bees to sit in the detergent solution for a minute or two before agitation — most of the mites will have by then dropped to the bottom of their own accord. Then agitate by swirling, not shaking.
To avoid peering through foam to count mites, use a 10x magnifying mirror (6-inch diameter) placed about 4 inches below the bottom of the cup. Remove the bees above the mites, so that you are looking up at a white background (the sky through the solution) (Figure 16).
Fig. 16 One of our crew, Thomas McCluskey, at the mite wash table with a magnifying mirror stand. We use color-coded cups with matching hive markers to keep track of which count goes with each hive (the temporary checklist in front of him helps greatly to avoid confusion, as we routinely perform more than 50 mite washes at a sitting). We’ve developed a tried and true system, the details of which I’m happy to share with other beekeepers and researchers.
Being a penny-pinching beekeeper, I reuse the Dawn solution so long as it is foamy. It gets cloudy, but that’s not a problem if you are counting mites looking from the bottom up. I simply pour the used solution through a tea strainer to remove the mites.
Practical application: While performing mite washes today, my son Eric told me today that he prefers the scent of Dawn Lemon Essence over Lavender. I’m sure that this will be yet another subject of debate for beekeepers.
It takes only minutes to monitor your hives for varroa. You’ll enjoy healthier and more productive colonies if you maintain a low level of this virus vector in your colonies at all times.
Updates: Mites drop more slowly in detergent/water, so if you use my two-cup design for mite washing, be sure to lift the screened cup out slowly. We find that most mites have already dropped within a minute, even without agitation. I have not yet had the opportunity to quantify how little agitation is actually required.
References
[1] I’ve recently updated the model to make it more user friendly. Download it at https://scientificbeekeeping.com/randys-varroa-model/
[2] https://arstechnica.com/science/2020/02/physicists-determine-the-optimal-soap-recipe-for-blowing-gigantic-bubbles/
[3] De Jong, D, et al (1982) A comparative analysis of shaking solutions for the detection of Varroa jacobsoni on adult honeybees. Apidologie 13(3): 297-306.
[4] Rinderer, TE, et al (2004) Re-examination of the accuracy of a detergent solution for varroa mite detection. American Bee Journal 144(7):560-562.
[5] They are both anionic surfactants, having a negatively-charged oxygen molecule from the sulfate at the end of a carbon chain. Although they work well to “dissolve” fats into water, I suspect that it is their foaming action that is important.
[6] Bondi, CAM, et al (2015) Human and environmental toxicity of sodium lauryl sulfate (SLS): Evidence for safe use in household cleaning products. Environ Health Insights 9: 27–32. doi: 10.4137/EHI.S31765
[7] https://scientificbeekeeping.com/an-improved-but-not-yet-perfect-varroa-mite-washer/
[8] https://extension.colostate.edu/topic-areas/insects/insect-control-soaps-and-detergents-5-547/
[9] Ewan M Campbell, EW, et al (2010) Gene-knockdown in the honey bee mite Varroa destructor by a non-invasive approach: studies on a glutathione S-transferase. Parasites & Vectors 3: 73.
[10] Vesco, U & G Guido (2014) Sugar shaking for varroa monitoring: verifying repeatability, mite recovery rate and bee sample precision. 3rd World Symposium of Organic Beekeeping.
[11] Macedo, P, J Wu & M Ellis (2002) Using inert dusts to detect and assess varroa infestations in honey bee colonies, Journal of Apicultural Research, 41(1-2): 3-7.
[12] Flores, JM, et al (2015 ) Reliability of the main field diagnostic methods of Varroa in honey bee colonies. Archivos de Zootecnia 64(246): 161-165.
[13] Thanks to Dr. David De Jong for personal communication regarding details of his study.