2022 Extended-release Oxalic (OAE) Update Part 2
An additional test of matrices and OA:gly ratios. 1
Cardboard Strips vs. Sponges. 3
Compostability of spent sponges. 3
A possible easy solution for getting us legal 13
2022 Extended-release Oxalic (OAE) Update
Part 2
First published in ABJ April 2022
Randy Oliver
ScientificBeekeeping.com
I continue with my research on various application methods of extended-release oxalic acid (OAE), and my efforts to get the method registered with EPA.
An additional test of matrices and OA:gly ratios
I get suggestions from all over the world for absorbent matrices to test. So come autumn, I rustled up enough “leftover” colonies with elevated mite counts to run a small comparative trial. I wanted to compare Maximizer strips to ShamWow towels, and to New Zealand chipboard (cardboard) hung strips. In addition, since New Zealanders typically use a higher-glycerin 1:1.5 ratio, I gave that ratio a try on both the hung chipboard and the Maximizer strips.
The colonies were in double deeps, in poor shape due to dearth and mites, with 5-10-frame clusters. With help from visiting beekeepers Jim Veitch, Jennifer Radke, and Catherine Edwards, we applied the test strips and fed each colony a patty of pollen sub. Each hive got four 1¾“ x 7½” strips of Maximizer (lightweight) or ShamWow rayon microfiber cloth, laid across the top bars, or three chipboard strips (the recommended amount) hung over the top bars in each brood chamber (Figure 1). The treatments were assigned in a randomized block design, blocked by starting mite count.

Fig. 1 I used Beequip chipboard strips from New Zealand, soaked in a 1:1.5 ratio of OA:gly, by weight. New Zealand supply houses carry a variety of such precut strips.
I ran the trial for 77 days, taking mite wash counts at start and finish (Table 1).
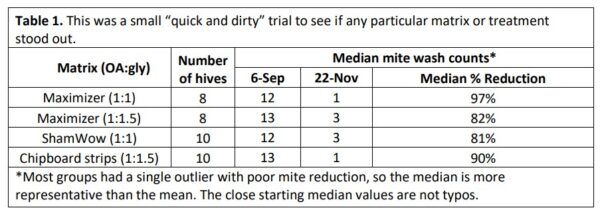
Practical application: All the tested matrices exhibited good mite reduction, with the Maximizer 1:1 and chipboard 1:1.5 strips having the most ending counts of zero or 1. Although Maximizers at the 1:1 ratio looked best (in either raw or worked data), I would like to test chipboard strips at the 1:1 ratio.
Chipboard Strips vs. Sponges
I’ve been focusing upon absorbent pads that can be quickly laid across the top bars of the lower brood chamber, rather than chipboard strips which need to be inserted between the frames of both brood chambers (which is far more time-consuming). But there are situations (such as in singles) in which there is not enough exposure area to the upper surface of a delivery pad to be effective, so the treatment would then need to be inserted between the frames. Chipboard strips are definitely easier to insert than are soft matrices such as sponges or Maximizers.
Practical application: One would expect that strips hung between the frames would have more contact with the bees’ bodies as they squeeze by, and thus be more effective at distributing the OA/gly onto the workers. Surprisingly, that does not appear to be the case. For full efficacy of OAE with pads laid across the top bars between the brood chambers, it takes about 55 sq in (355 cm2) of matrix surface area, holding 50 g of OA dissolved in 50 g of glycerin, per double-deep hive. For cardboard strips hung over the frames, based upon data from Aluen CAP, Dan Aurell, the New Zealand beekeepers, and my own, it actually requires more surface area when using strips (80-115 sq in/ 520 – 740 cm2 per double-deep hive). Go figure!
Compostability of spent sponges
Once an OAE matrix has largely dispensed its OA, it needs to be pulled or scraped out of the hive, since the bees generally don’t remove them. The spent strips still contain some amount of OA, and need to be handled appropriately during disposal. Some pieces invariably fall on the ground, so I favor using biodegradable strips, so that we don’t leave non-biodegradable plastic pollution in our yards (the ShamWows may not fit this bill [[1]]).
If a fallen strip gets hit by rain, the OA gets washed out, and the remaining cellulose gets treated by decomposition organisms similarly to that in a fallen leaf. I wondered whether a spent Swedish sponge still containing OA and glycerin would get decomposed if added to a compost pile. So I buried a few in an active compost pile in my garden (Figure 2).

Fig. 2 After a few weeks, I had trouble locating any remnants of the pink sponges, since they were well along in the process of being decomposed.
Practical application: I abhor the way that we’re polluting our environment with plastic, so prefer using biodegradable cellulose matrices.
A winter field trial
By this time, we’ve gotten a good idea about the amount of OAE pad required for good efficacy against varroa during the summer, when colonies have an active broodnest. I also had preliminary data that it could be efficacious during winter and didn’t appear to harm the colony. So I decided to run a full-scale trial in a yard at an elevation that “normally” gets cold enough to cause a winter brood break (as if there were any weather “normal” any more in California).
There were two questions that I wanted to answer:
- Would treatment with OAE cause any adverse effects upon the long-lived winter bees due to prolonged exposure to OA, or otherwise adversely affect colony buildup prior to almond pollination?
- Could OAE be used to try to zero out mites over winter, giving colonies a clean start for their first rounds of brood rearing?
Methods
In early November, following a rough summer dearth, we graded 45 moderately-strong double-deep colonies for strength and took mite wash counts (the colonies had been previously treated with OAE strips). Most had ceased brood rearing a week or so earlier (due to cool weather and lack of incoming pollen), and had only a small amount of sealed brood (about to emerge) remaining. I assigned treatment in a randomized block design, first blocking by mite infestation rate, and then by colony strength, and then randomly assigning an OAE treatment to one of each matched pair of hives. We applied ½ of a Swedish sponge (saturated with a solution of 25 g each of OA and glycerin [1:1 formulation by weight]) to each hive in the Test group (Figure 3).

Fig. 3 We applied a half OAE sponge to each Test colony, placing it in the front third of the cluster. Although this location resulted in suboptimal exposure of the bees to the treatment, it left room for us to feed pollen sub in the middle of the clusters.
Practical application: Bees won’t consume pollen sub that’s come in contact with an OAE sponge, so one should leave a space between them. Another tip that I’ve received is to insert a ¼-inch shim between the brood chambers in order to give the bees more space above the OAE sponges for them to walk over, but I have not yet tested this.
To our surprise (something that we’re getting used to), the weather unexpectedly warmed up, hitting 70°F on the first of December! Plants responded, the bees started bringing in a little pollen, and the colonies resumed rearing a bit of brood. I’ve laid out the timeline in Figure 4.
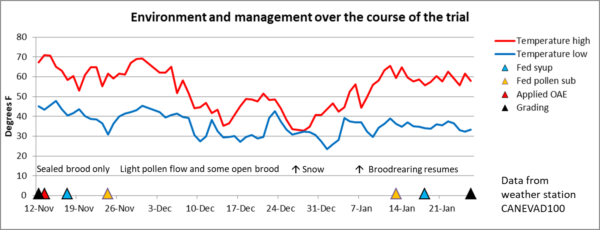
Fig. 4 The yard got slammed by a cold snowstorm on December 26, which really set back the “spring turnover” of the colonies. But by mid-January, the temperature rose, the alders started producing pollen, and the colonies resumed brood rearing in earnest.
The surprise subfreezing temperatures and coverage with snow hit the colonies hard, and many had piles of dead bees in front of them once the snow melted (Figure 5). We checked in front of every hive for the presence of dead bees, comparing it to treatment assignment, and did not observe any correlation with whether the colony had received OAE or not.
Practical application: The above observation suggests that OAE treatment did not cause elevated mortality of the aged adult bees.

Fig. 5 I took samples of bees from the nine worst piles, and took them home for microscopic analysis, looking for the presence of nosema spores in a homogenized subsample of 10 bees — three subsamples had no nosema, three had a little, and three were fairly well infected.
Practical application: It didn’t appear that nosema was the main problem, since I would have expected to see nosema in dead older bees following a late-winter pollen flow (since pollen in the gut stimulates nosema reproduction).
Then it warmed up again, and by late January the colonies were full of a new round of sealed brood (Figure 6).
 Fig. 6 I took this photo on the day of final grading. The cluster barely covered three frames, but was rearing as much brood as it could keep warm. There are about 1500 pupae on this side, and only 445 adult workers (yes, I counted ‘em). The 7-frame colonies contained three full frames of brood. It’s easy to see how colonies can grow quickly once pollen becomes available.
Fig. 6 I took this photo on the day of final grading. The cluster barely covered three frames, but was rearing as much brood as it could keep warm. There are about 1500 pupae on this side, and only 445 adult workers (yes, I counted ‘em). The 7-frame colonies contained three full frames of brood. It’s easy to see how colonies can grow quickly once pollen becomes available.
Although I would have liked to have performed one more grading after the sealed brood emerged, we needed to move the hives to almond pollination, so performed final grading on January 26.
Results
The raw data is presented in Figure 7.
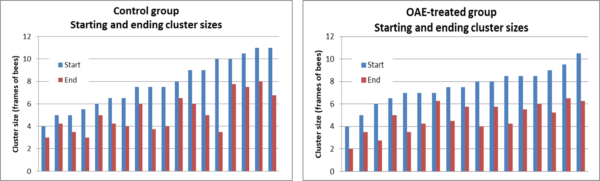
Fig. 7 None of the colonies had yet rebuilt to their starting strengths. For analysis (including the graphs above), I removed four badly-dwindled outlier colonies from the Control group, so did the same for the OAE group as well.
Rather than the absolute values shown above, it may be more informative to compare the colonies’ relative changes in strength vs. their starting strengths (Figure 8).

Fig. 8 All the colonies were weaker in January than in November, but since they were full of sealed brood, they would be expected to greatly increase their sizes to above starting strength by time of grading in almonds. The OAE-treated group as a whole lost slightly more strength than did the Controls, but the difference was not statistically significant [[2]].
Practical application: So was there an adverse effect from treatment? It’s difficult to tease out from this small data set (with only one replicate). I now wish that I had prioritized colony strength (over initial mite count) when I assigned treatments, since larger clusters have a distinct advantage over smaller clusters as they initiate brood rearing [[3]]. As you can see in Fig. 7, the Control colonies wound up starting out stronger than the Test group, and thus would have been expected to overwinter and then grow better than the Test colonies. So I’m fairly confident that there was no adverse effect upon colony strength or buildup.
So far I haven’t discussed the possible benefit from treating with OAE in the first place — did it bring down the mite infestation rates? I fully expected over-winter treatment to zero out the varroa infestation rate, but it didn’t (Figure 9).
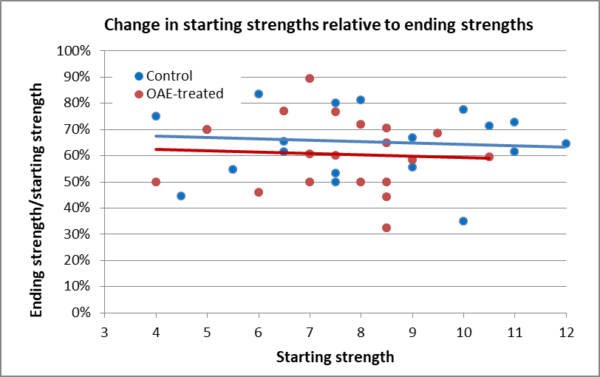
Fig. 9 The mite wash counts generally grew in the Control colonies over winter, but to my surprise, having an OAE pad in the hive did not zero out the infestation rates of the Treated group.
I was surprised by the lack of efficacy due to treatment after 71 days of exposure. The average mite count did go up by 55% in the Control group, but only decreased by a measly 3% in the treated group (Figure 10).
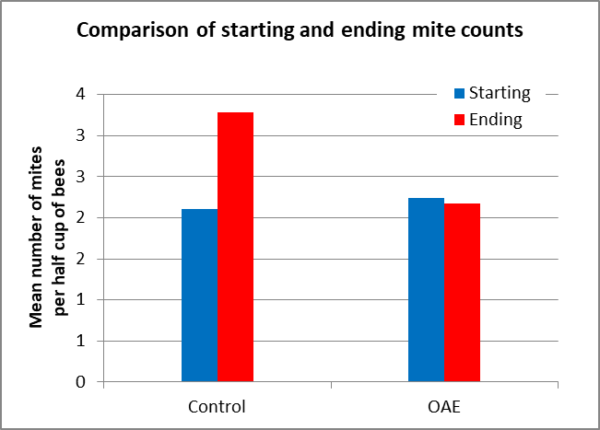
Fig. 10 I used the Henderson-Tilton formula to calculate average efficacy of the treatment, which worked out to only a disappointing 37%.
Discussion
Practical application: Although OAE appears not to cause harm to a colony over winter, it may not cause much harm to the varroa mites either! Although OAE can be highly efficacious during the summer, it remains to be determined whether it is appropriate for winter treatment.
The Elephant in the Room — our “scofflaw problem”
After the invasion of the varroa mite, researchers around the world published research on how this or that agricultural miticide (e.g., Mavrik, coumaphos, Taktic) could successfully be used to control varroa. Unfortunately, due to the frustratingly-slow registration process required by the EPA to register a product for use in bee hives, beekeepers, desperate to keep their colonies alive, would start using such treatments prior to approved formulated products being brought to the market [[4]].
Such “off-label” use of miticides is in violation of the law, and even when a legal product was finally brought to the market, many beekeepers continued to use the cheaper homemade treatments. It is no secret that our commercial industry remains widely dependent upon imported, unregistered, illegally-applied amitraz products for mite control. The fact that beekeepers, who often demand pesticide application compliance by farmers, are often pesticide scofflaws themselves, is a point not lost upon the EPA and its enforcement agents.
Our state apiary inspectors (as well as the EPA state lead agencies) have long turned a blind eye towards the illegal use of homemade miticide treatments for varroa management.
Practical application: Some inspectors are getting tired of this hypocrisy, and are calling upon the EPA to start enforcement actions. Not only that, but mites are finally starting to develop resistance to amitraz. Because of this, commercial beekeepers are looking for another effective way to manage varroa.
It occurs to me that now is a chance to get our industry back into compliance. One of the most promising miticides to replace amitraz is oxalic acid, for which there is only one currently registered (and high-priced) product, with only three approved application methods. What I was trying to do was to get the Registrant — USDA-ARS — to get the extended-release (in glycerin) application method added to the label (as well as some other improvements) Unfortunately, USDA recently decided to cease pursuing new registrations. A number of beekeepers have told me that they are disappointed in USDA-ARS, which hands out millions of dollars in grants to bee researchers each year, for not helping our industry by getting this additional application method approved while they were still the Registrant. So I’m back in discussion with EPA and others.
Reality Check
OAE is currently being researched in many countries. A formulated product — Aluen CAP — is registered for use in Argentina, Uruguay and Chile, and should soon be approved in other countries. In other regions, some beekeepers are jumping the gun and mixing their own, and due to its obvious safety, authorities often turn a blind eye to this practice. Generic 99.6% purity OA is readily available, meaning that no matter the law, many beekeepers will likely mix up their own OA treatments, rather than purchasing a pricey formulated registered product. What can we do in order to get us into compliance with the law?
Practical application: There is only a small profit margin of in commercial beekeeping, so professional beekeepers try to avoid any unnecessary costs. This means that there is a strong financial disincentive to purchase high-priced registered miticides. Thus, the use of inexpensive illegal treatments results in an unlevel playing field, giving those who use unapproved treatments an unfair competitive advantage over those professional beekeepers willing to use only approved mite treatments. I don’t want to break the law, but I also don’t want to pay through the nose for a registered oxalic product.
A possible easy solution for getting us legal
New Zealand’s Ministry for Primary Industries came up with a very workable solution. They allow beekeepers to prepare and apply generic oxalic acid to hives that they own, plus take full responsibility for applicator safety (Figure 11).
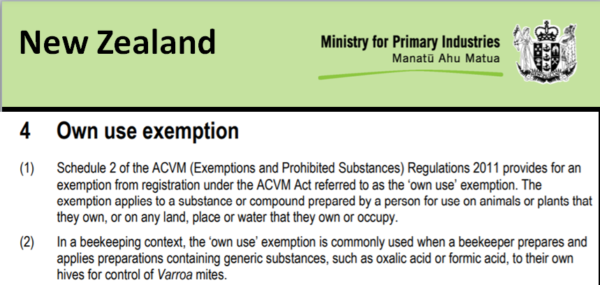
Fig. 11 I lifted the above snip from the actual document [[5]]. Beekeepers in New Zealand can legally prepare and apply generic oxalic and formic acids to their own colonies. This is a sensible solution, since neither substance poses “any unreasonable risk to man or the environment, or a human dietary risk from residues that result from a use of the pesticide” [[6]]
The Exemption does not allow the sale of any unregistered formulated product, but supply houses can sell the raw ingredients for beekeepers to mix up their own (Figure 12).

Fig. 12 This is a kit of the raw materials for making OAE strips, sold by Beequip.nz in New Zealand. Although a beekeeper is allowed to make them for their own use, selling a formulated product would require registration with New Zealand’s Environmental Protection Authority.
If EPA were to grant an Own Use Exemption, the Agency would be off the hook as far any safety responsibility or regulation of use of generic OA by beekeepers, and only be responsible for the registration and regulation of formulated products. This would be of huge benefit to beekeepers, since they would then be able to legally use inexpensive generic OA, and the State Lead Agencies would no longer need to turn a blind eye toward the practice. This could be a win-win for both the beekeeping industry and the EPA.
Unfortunately, in a recent meeting that I requested, EPA’s Office of Pesticide Programs was not open to granting us an Exemption. I’m not sure whether we’d get any traction by having our national organizations petition them. So I’m currently in discussion with some individual beekeepers about pursuing us becoming a Registrant in order to get the extended-release (in glycerin) application method approved, so that we could provide inexpensive “registered” OA, with the OAE application method on the label, to the beekeeping supply houses. Stay tuned — I may start a GoFundMe fundraiser to allow us beekeepers to legally pursue a solution to our “scofflaw problem.”
To be continued…
Citations and notes
[1] ShamWow is made of a blend of rayon and polypropylene. Rayon is a manufactured regenerated cellulose fiber. Polypropylene is a non-biodegradable plastic.
[2] Mann-Whitney one-tailed U-value is 119. The critical value of U at p < .05 is 102. Therefore, the result is not significant at p < .05. The z-score is 1.10566. The p-value is .1335. The result is not significant at p < .05.
[3] Nolan, WJ (1932) The development of package-bee colonies. USDA Technical Bulletin No. 309.
[4] Delaplane, K (1997) Practical science—research helping beekeepers 3. Varroa. Bee World 78(4): 155-164.
[5] Advertising and own use guidance for compounds for management of disease in beehives (mpi.govt.nz)
[6] Summary of the Federal Insecticide, Fungicide, and Rodenticide Act | US EPA



