2022 Extended-release Oxalic Update Part 3
Contents
You don’t need to kill a single mite in order to control varroa! 5
Varroa’s world: Olfaction, taste, and touch. 7
Disruption of mite sensory perception. 9
2022 Extended-release Oxalic Update
Part 3
First published in ABJ May 2022
Randy Oliver
ScientificBeekeeping.com
Although I’ve worked extensively with extended-release oxalic acid (OAE) for the past several years, there’s still plenty more to learn about this potentially game-changing treatment for varroa.
I’ve been amazed by how a single application of a pad containing OA dissolved in glycerin, given to a colony badly infested with varroa, can with time completely zero out its mite count, with no apparent adverse effects upon the colony. This observation raises the questions of exactly how the treatment works (mode of action), and why it takes so long.
The slow effect of OaE
There’s no question that oxalic acid (OA) is able to kill mites. An application by dribble (OAD) or vapor (OAV) can cause a rapid spike in varroa mortality, as evidenced by the drop of dead and dying mites onto a stickyboard. But that effect largely wears off after three days. On the other hand, an application of extended-release OA in glycerin (OAE) takes a little longer to ramp up the mite drop, and that elevated drop rate then extends for about a month. Let’s look a little deeper into this difference.
I’ve received data sets of mite drop counts after OAV from a number of beekeepers, Donald Aiken’s being representative (Figure 1).
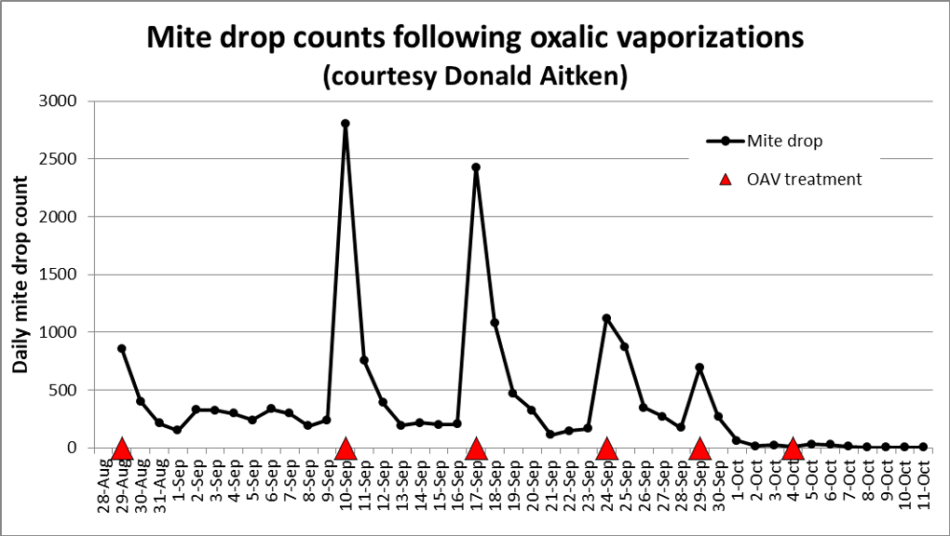
Fig. 1 Aiken recorded the daily counts of fallen mites following six OAV applications. Note that the daily mite drop immediately spikes upward in the first 24 hours after treatment, and then quickly tapers off over the next three days — despite the continual emergence of mites from the brood each day. This pattern is typical, and indicates a short-term residual action of OAV.
Compare the above to data to that for OAE strips (Figure 2).
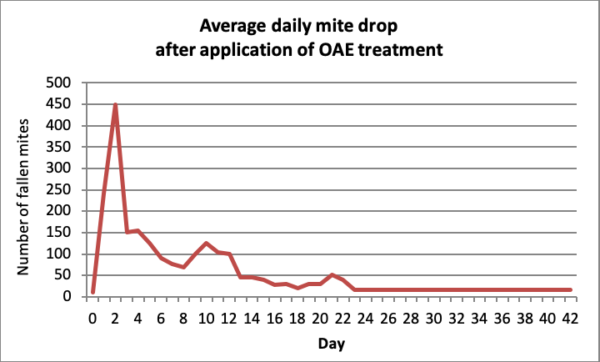
Fig. 2 Dehaibes [[i]] applied Aluen CAP extended-release OA strips to 19 colonies. The graph above shows the average daily mite drop [[ii]]. Unlike the short-term spike observed immediately following an OAV or dribble, an application of OAE takes a few days to exhibit full effect, but then continues to show residual activity for up to a month.
Practical application and question: My own limited data (not shown) closely reflect that of Dehaibes, including the two bumps roughly ten days post-application. (I’m currently working on modeling to figure out why this occurs.) However, with the lower-glycerin ratio that I use, the proportional increase in mite drop is lower than that from the strips that they used. But the big question is, if increased mite drop occurs for only a month, then why does it take two months to attain full efficacy?
Mite turnover
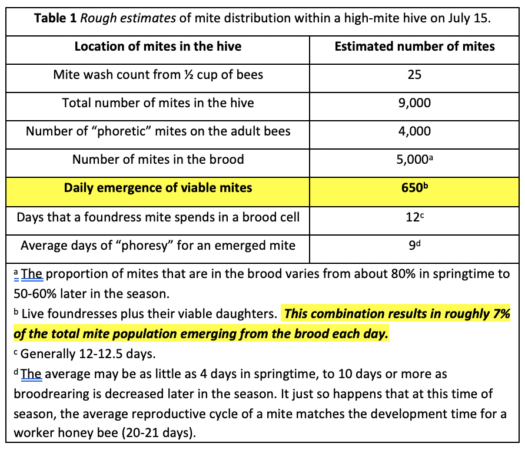
When we look into the exposure of mites in the hive to the applied oxalic acid, we must take into account the daily “turnover” of mites as they rotate into the brood for reproduction, and then emerge ~12 days later to disperse (the “phoretic” phase). So let’s put the above mite drop graphs into perspective by looking at some numbers for a high-mite colony (much higher than Dehaibes’ hives) in mid-July, estimated by using my mite model [[iii]] (Table 1).
[i] Dehaibes, S, et (2020) Control of Varroa destructor development in Africanized Apis mellifera honeybees using Aluen Cap (oxalic acid formulation). International Journal of Acarology. 46(6): 405-408.
[ii] I scaled the numbers off Dehaibes’ graph, and took the liberty of using the counts from their Control group as a baseline value.
[iii] From https://scientificbeekeeping.com/randys-varroa-model/
Practical application: The above figures indicate that in a high-mite late-summer colony, there is a turnover of around 650 mites emerging from the brood each day. This helps to explain why an oxalic vaporization treatment can cause a very high mite drop for a few days, since those emerging mites are being newly exposed to the treatment. But the residual activity of the vaporization treatment quickly wears off. Compared to OAV, the slow release of OAE would be expected to cause a lower initial mite drop, but result in an longer-term elevated rate of mite drop as mites emerge from the brood day after day.
The data from Dehaibes and Maggi [[i]] indicate that only around 70% of the mites in a hive drop during the first two weeks of treatment with OAE, despite the fact that any mites in the brood would have rotated out onto the adult bees during that period. Curious, I graphed out the effect upon a colony’s total mite population from applying a treatment that killed a certain percentage of only the ever-rotating population of “phoretic” mites each day (Figure 3).
[i] Maggi, M, et al (2015) A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie 47(4): 596-605.
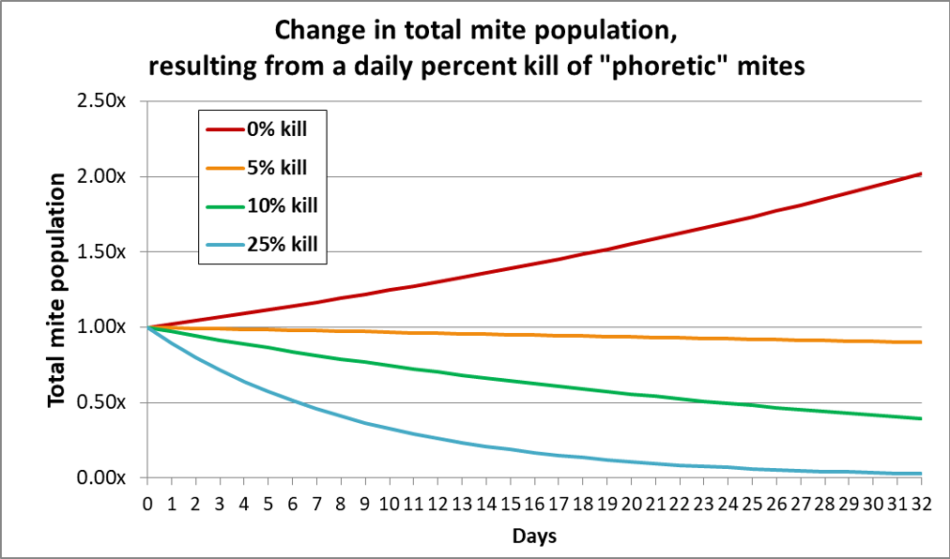
Fig. 3 With zero kill of the phoretic mites, the overall varroa population would double in a month. Killing 5% of the phoretic mites each day arrests varroa population increase. But it takes killing 25% of the temporarily phoretic mites each day to eliminate most of a colony’s mite population within a month. Note that the 25% kill rate plot closely matches the 70% reduction at two weeks recorded by Dehaibes.
Practical application: With the “gentler” and slower-acting 1:1 ratio of OA to glycerin that I’ve mostly tested, the kill rate appears to be lower, since it takes at least two full months to attain full mite reduction. The tradeoff is that a high-glycerin ratio appears to cause a more rapid mite reduction, but may have more adverse effects upon the bees and brood (as well as being messier to handle).
The above kill calculations appear to explain everything, other than one bothersome question …
A bothersome question
This brings me back again to my observation that the infestation rate (as measured by alcohol wash) of the adult bees actually increases during the first month of treatment, despite the elevated rate of mite mortality (as indicated by mite drop). If the treatment were actually killing 25% of the exposed mites every day, we’d expect to see the infestation rate of the adult bees quickly decline, since a daily 25% kill far exceeds the expected 7% daily emergence of replacement mites from the brood.
This makes me wonder whether OAE is affecting the mites in some manner that prevents them from successfully reentering brood cells to reproduce. And this brings us to the concept that:
You don’t need to kill a single mite in order to control varroa!
Practical application: All mites will eventually die of old age. Unless their reproductive rate is greater than their rate of natural mortality, their population cannot increase.
To control varroa, all that you need to do is to halve their success at reproduction (Figure 4).
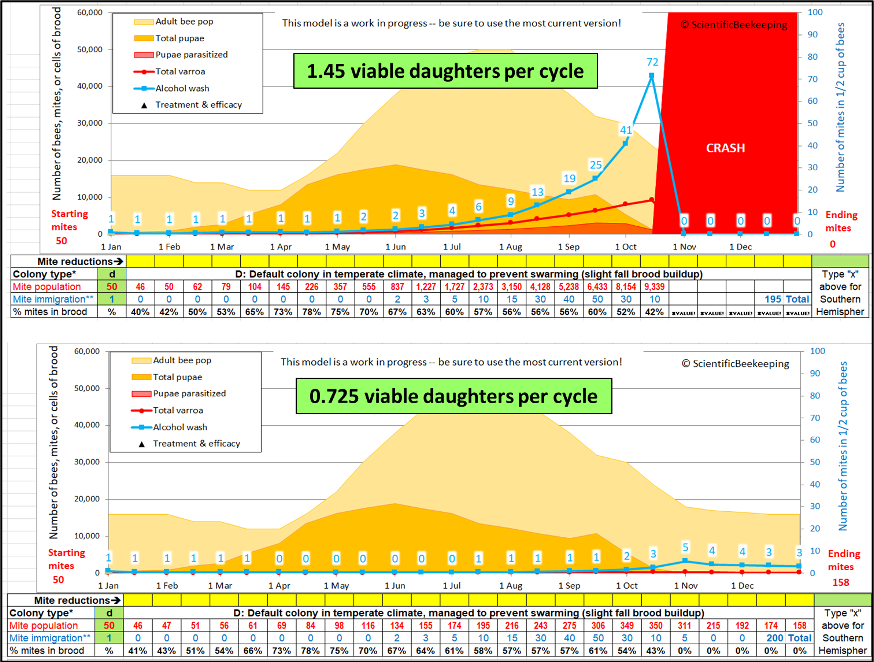
Fig. 4 I ran two simulations, with identical inputs, other than reducing the average reproductive success of foundress mites in worker brood cells (indicated in the green text boxes). Note that in the lower simulation, in which I reduced the mites’ reproductive success by half, varroa never became an issue.
Note: Although the mite population in the lower half-rate simulation did end up higher than the starting count by the end of the year, additional runs indicated that at the lower rate of reproduction, it would take 3-4 years for the mite population to build up high enough to cause colony death.
Practical application: It may be constructive to not limit ourselves to treatments that result in the immediate killing of adult mites, and consider long-term treatments that hamper the mites’ ability to successfully reproduce.
Could OAE, due to affecting the mites’ sensory papillae, be hampering them from reentering the safety of brood cells, which would perhaps result in (1) more chance of exposure to a fatal dose of OA, and/or (2) lessen their ability to successfully reproduce? Could those combined effects then be the reason that OAE (1) takes so long to reduce the infestation rate on the adult bees, but (2) is able to eventually zero it out completely?
So how might this work?
Varroa’s world: Olfaction, taste, and touch
Varroa mites are adapted to live in total darkness, and lack eyes. They are completely dependent upon their senses of smell (olfactory), taste (gustatory), and touch (tactile/mechano-reception), as well as for temperature and humidity (thermo- and hydro-reception) in order to not only identify suitable adult bees to catch a ride on, but also for each step involved in the critical recognition of a suitable cell containing a 5th-instar larva to hop into for reproduction.
But mites also lack antennae. Instead, they employ only six of their eight legs for walking, and use their two longer front legs similar to how insects use their antennae. On the tips of those front legs are delicate sensory papillae, through which they experience their world (Figure 5). They have additional sensory papillae on their pedipalps, mouthparts, and other places on their bodies.
Glossary: papilla — a small protuberance; if hair-shaped, called a sensillum.

Fig. 5 I snapped this shot of the underside of a mite’s front leg. The balloon-like inflatable and sticky empodium is to the upper right. Below that, at the tip of the leg, is the “sensory pit organ,” consisting of nine internal papillae, with nine longer hair sensilla surrounding the organ. Some of the sensilla are “wall-pore sensilla,” presumably for the perception of volatiles. Other sensilla are “non-pore sensilla” which serve as tactile, hygro- or thermo-receptors, whereas the morphology of a third type indicates a gustatory function [[i],[ii]]. You can view far more detailed scanning electron micrographs of these organs at [[iii], [iv], [v]].
Practical application: If a treatment affects the function of these sensitive papillae, it would be like blinding a mite and holding its nose, leaving it relatively helpless.
Adult mites outside of a brood cell face a dangerous environment. A mite needs to quickly identify a nurse bee [[vi]], so that it can feed on the nurse’s well-developed fat bodies while it hitches a ride, in order to develop its own ovary and first egg (this ride also allows time for the sperm that the mite received from her brother to mature). A mite can rapidly scamper from bee to bee, but must avoid being bitten or groomed off, so it must somehow locate a space between the abdominal sternites of its unwilling mount, where it can dig in and safely spend its time while it enjoys a meal.
But it can’t stay dug in forever — once her ovary is developed, the mite needs to reproduce, so as the nurse bee carries her from cell to cell as she is smelling and feeding larvae, the mite is simultaneously “sniffing” for the kairomonal signal indicating whether the cell contains a 5th-instar larva calling to be capped over. The mite must then quickly jump off the nurse and onto its new “reproductive host” (soon to be a pupa). Thus in general, a mite is generally opportunistic, waiting for the nurse that it’s riding on to stick her head into a cell containing a 5th-instar larva. However, a mite may on occasion dismount from its ride to actively seek a cell (questing behavior). The way that it finds its way around is well described by Dillier [[vii]]:
Varroa uses its two front legs in the same way as insects use their antennae. These legs are only rarely used for movement and are more frequently displayed in the air.
During many hours of observation of brood frames to collect infested , we repeatedly observed mites leaving nurse bees and running several centimetres over the comb surface before disappearing. [Another researcher] regularly saw Varroa mites walking on undisturbed comb surfaces, … walking along open cell rims, often dipping into the lumen of a cell and then alternate to the other side to dip into the lumen of the next cell and so on.
Practical application: Any beekeeper who’s spent much time looking at combs of mite-infested colonies, soon realizes how rare it is to actually observe a mite walking over a comb surface (or even on the back of a bee). Most all the mites in a hive are either hidden on the bellies of nurse bees, or safely ensconced in a brood cell. It’s when they are forced to move about in the colony that they are most vulnerable.
The mite also has another challenge. If at some point the nurse bee that it’s riding on transitions to mid-age or foraging behavior, the bee’s cuticular hydrocarbon smell changes, which cues the mite that it must then identify a new nurse, and then take the risk of moving from the bee that it’s riding on to a new (and again unwilling) ride in order to have a chance of being carried into a brood cell.
Practical application: Does treatment with OAE somehow interfere with the mites’ ability to identify the right age of bees to hitch rides on, or the right cells to invade?
Disruption of mite sensory perception
I’m hardly the only one who’s thought of this. It’s also occurred to other researchers, notably Dr. Victoria Soroker and her associates, that we might be able to come up with treatments that disrupt the mites’ sensory perception, and thus their ability to successfully get around and reproduce [[viii]]:
Hive volatiles, mainly from adult bees and brood, play a crucial role in the parasite’s life cycle, by guiding host finding, selection and regulating its reproduction, suggesting that the mite’s olfaction may be an important target for new specific control agents … Inhibition of host sensing leads to incorrect Varroa host selection or reduction in mite’s ability to reach a host.
Practical application: If we could find a sustained-release treatment that hampered the ability of a mite to get around in the hive and locate 5th-instar larvae, this might be the Achilles’ heel of varroa that we’ve been looking for.
But wouldn’t olfactory disruption also affect the bees also living within the darkness of the hive? Perhaps not. Mites are not closely related to insects, and use some different “chemosensory or odor-binding proteins” than do honey bees [[ix], [x]].
Practical application: There’s a chance that we might identify treatments that affect varroa odor-binding proteins, but not those of the bees. This is a question when we apply treatments, such as thymol, formic, or oxalic acids, that affect cell membranes. Do these strong chemicals affect the sensory perception of mites (which would help us) or the bees (which may be harm the colony), and if so, do they affect one more than the other?
“Blinding” or irritant?
Let’s get back to oxalic acid. It can clearly exhibit acute toxicity to varroa. It’s possible that a sublethal dose might affect mite olfaction. But could it perhaps exhibit a simple irritant effect upon the mite that disrupts their movement?
Could the light “dressing” of OA onto bees’ bodies from either OAV or OAE (which doesn’t appear to be bothersome to bees) irritate the mite’s delicate footpads (empodia)? Could it be that having a tiny amount of OA on a bee’s body “hairs” thus make a mite hesitant to come out of its “safe spot” between the abdominal plates to walk about?
Practical application: Varroa mites must routinely “rotate” between the “phoretic” phase on adult bees, to the “reproductive” phase in capped brood, as well as rotating off its ride as she ages. Could OAE somehow disrupt this rotation? We could use more research along this avenue!
A relevant question
Beekeeper Jeff Steenbergen asked me whether “the presence OA crystals has an off-gassing type effect on mite reproduction, or if the bees really need to move it around the hive?” I started to answer that that OA wouldn’t evaporate at hive temperature. But then I remembered that (to my surprise) I previously found that Apivar strips unexpectedly appear to exhibit vapor action [[xi]].
So I duplicated the setup that I used to test for vapor action of amitraz, by placing some mite-infested bees into two cup cages, one with a layer of OA crystals separated from the bees (and mites) by a screen, and placed them in an incubator at 80°F, 65% RH, in darkness, for three days, feeding 1:1 sucrose (Figure 6).
[i] Rosenkranz, P, et al (2010) “Biology and control of Varroa destructor.” Journal of Invertebrate Pathology 103: 96-119.
[ii] Singh, N, et al (2016) Identification and gene-silencing of a putative odorant receptor transcription factor in Varroa destructor: possible role in olfaction. Insect Mol. Biol. 25: 181-190.
[iii] Dillier, F-X, et al (2006) Review of the orientation behaviour in the bee parasitic mite Varroa destructor: Sensory equipment and cell invasion behaviour. Revue suisse de zoologie; annales de la Société zoologique suisse et du Muséum d’histoire naturelle de Genève 113(4):857-877
[iv] Soler, MD, et al (2005) Scanning electron microscopy of foreleg tarsal sense organs of the poultry red mite, Dermanyssus gallinae (DeGeer) (Acari : Dermanyssidae). Micron 36(5):415-21.
[v] Iovinella, I, et al (2018) Proteomic analysis of chemosensory organs in the honey bee parasite Varroa destructor: A comprehensive examination of the potential carriers for Semiochemicals. Journal of Proteomics 181: 131-141.
[vi] Xie, X, et al (2016) Why do Varroa mites prefer nurse bees? Scientific Reports 6: 28228.
[vii] Dillier, F-X, et al (2006) op cit.
[viii] Soroker, V, et al (2019) Olfaction as a target for control of honeybee parasite mite Varroa destructor. In: Picimbon, JF. (eds) Olfactory Concepts of Insect Control – Alternative to insecticides. https://doi.org/10.1007/978-3-030-05060-3_6
[ix] Iovinella (2018) op cit.
[x] Eliash, N, et al (2019) Varroa chemosensory proteins: some are conserved across Arthropoda but others are arachnid specific. Insect Mol Biol. 28(3): 321-341.
[xi] Questions on Amitraz. https://scientificbeekeeping.com/6501-2/

Fig. 6 You can see the OA crystals on the bottom of the Test cup to the left. After 3 days, zero mites had dropped in either cage. A mite wash afterwards confirmed that there were still live mites on the bees in both Test and Control cups. This non-replicated “quick and dirty” test didn’t directly answer whether reproduction of the mites might be affected by vapor action, but presence of OA near the bees certainly didn’t cause the mites to fall off.
Practical application: The above quickie test (performed during winter when it was difficult to find an infested colony) suggests that OA crystals don’t exhibit vapor action upon adult mites. However, it’s possible that the oxalic esters formed when the acid is dissolved in glycerin, or the trace amounts of OA that I can titrate off the outside of bees’ bodies, exhibit some sort of olfactory-disruptive, or foot-irritant properties to the mites. I’ll leave it to some postdoc to run a proper study to determine whether OAE might affect mite reproductive success.
Wrap-Up
As far as I’m concerned, we’re still low on the learning curve on how best to apply and utilize OAE, and in understanding its actual mode(s) of action. I appreciate feedback from other researchers and permitted beekeepers working with it!
News flash: EPA’s position on experimental use of EOA
I have received numerous requests from “citizen-science” beekeepers across the country, wanting to run their own field trials of extended-release oxalic acid in their particular environments. For my research, I obtain a “Pesticide Research Authorization” from my own State Lead Agency each year, but other beekeepers have reported that their respective SLA refers them to EPA to obtain an “Experimental Use Permit” (EUP).
Gina Burnett, Senior Regulatory Advisor for the EPA’s Biochemical Pesticides Branch, was gracious enough to go over the regulations with me (relevant verbiage highlighted):
§ 172.3 Scope of requirement.
(a) An experimental use permit (EUP) is generally required for testing of any unregistered pesticide or any registered pesticide being tested for an unregistered use. However, as described in paragraph (b) of this section, certain of such tests are presumed not to involve unreasonable adverse effects and, therefore, do not require an EUP.
(b) Except as provided in subpart C of this part or as specifically determined by the Environmental Protection Agency (EPA), it may be presumed that EUPs are not required when:
(1) The experimental use of the pesticide is limited to:
(i) Laboratory or greenhouse tests,
(ii) Limited replicated field trials as described in paragraph (c) of this section to confirm such tests, or
(iii) Other tests as described in paragraph (c) of this section whose purpose is only to assess the pesticide’s potential efficacy, toxicity, or other properties.
(2) The producer, applicator, or any other person conducting the test does not expect to receive any benefit in pest control from the pesticide’s use.
(c) For purposes of paragraphs (b)(1)(ii) and (b)(1)(iii) of this section, the following types of experimental tests are presumed not to need an EUP:
(1) A small-scale test involving use of a particular pesticide that is conducted on a cumulative total of no more than 10 acres of land per pest, except that:
(i) When testing for more than one target pest occurs at the same time and in the same locality, the 10 acre limitation shall encompass all of the target pests.
(ii) Any food or feed crops involved in, or affected by, such tests (including, but not limited to, crops subsequently grown on such land which may reasonably be expected to contain residues of the tested pesticides) shall be destroyed or consumed only by experimental animals unless an appropriate tolerance or exemption from a tolerance has been established under the Federal Food, Drug, and Cosmetic Act (FFDCA) for residues of the pesticide.
Since the FDA has ruled that “Residues of oxalic acid in or on honey and honeycomb are exempted from the requirement of a tolerance when oxalic acid is used as a miticide in honeybee hives,” the EPA does not have any restrictions as to whether the honey can be harvested and consumed.
Bottom line: Unless your State has more restrictive requirements, you would not need to obtain an EUP from EPA to run small-scale trials with oxalic acid. Be clear that this only applies to use for experimental testing!
Citations and notes
[1] Dehaibes, S, et (2020) Control of Varroa destructor development in Africanized Apis mellifera honeybees using Aluen Cap (oxalic acid formulation). International Journal of Acarology. 46(6): 405-408.
[2] I scaled the numbers off Dehaibes’ graph, and took the liberty of using the counts from their Control group as a baseline value.
3] From https://scientificbeekeeping.com/randys-varroa-model/
[4] Maggi, M, et al (2015) A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie 47(4): 596-605.
[5] Rosenkranz, P, et al (2010) “Biology and control of Varroa destructor.” Journal of Invertebrate Pathology 103: 96-119.
[6] Singh, N, et al (2016) Identification and gene-silencing of a putative odorant receptor transcription factor in Varroa destructor: possible role in olfaction. Insect Mol. Biol. 25: 181-190.
[7] Dillier, F-X, et al (2006) Review of the orientation behaviour in the bee parasitic mite Varroa destructor: Sensory equipment and cell invasion behaviour. Revue suisse de zoologie; annales de la Société zoologique suisse et du Muséum d’histoire naturelle de Genève 113(4):857-877
[8] Soler, MD, et al (2005) Scanning electron microscopy of foreleg tarsal sense organs of the poultry red mite, Dermanyssus gallinae (DeGeer) (Acari : Dermanyssidae). Micron 36(5):415-21.
[9] Iovinella, I, et al (2018) Proteomic analysis of chemosensory organs in the honey bee parasite Varroa destructor: A comprehensive examination of the potential carriers for Semiochemicals. Journal of Proteomics 181: 131-141.
[10] Xie, X, et al (2016) Why do Varroa mites prefer nurse bees? Scientific Reports 6: 28228.
[11] Dillier, F-X, et al (2006) op cit.
[12] Soroker, V, et al (2019) Olfaction as a target for control of honeybee parasite mite Varroa destructor. In: Picimbon, JF. (eds) Olfactory Concepts of Insect Control – Alternative to insecticides. https://doi.org/10.1007/978-3-030-05060-3_6
[13] Iovinella (2018) op cit.
[14] Eliash, N, et al (2019) Varroa chemosensory proteins: some are conserved across Arthropoda but others are arachnid specific. Insect Mol Biol. 28(3): 321-341.
[15] Questions on Amitraz. https://scientificbeekeeping.com/6501-2/



